Fig. 2.
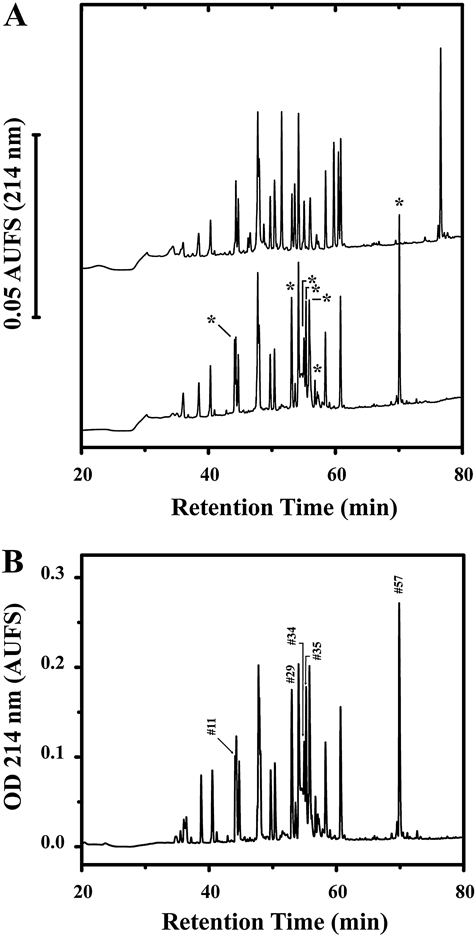
Identification and purification of disulphide-bonded peptides from a tryptic digest of Pf332 DBL domain.
A. Comparison of the analytical RP-HPLC elution profiles for DTT-reduced and non-reduced tryptic digest of refolded Pf332 DBL domain. Reduced (upper trace) and non-reduced (lower trace) digests (20 μg) were fractionated on a Vydac C18 (4.6 mm inner diameter × 250 mm) column under identical chromatographic conditions. The column was developed at a flow rate of 1 ml min−1 with a linear 120 min gradient from 0 to 70% buffer B, where buffer A was 0.05% (v/v) trifluoroacetic acid in Milli Q water, and buffer B was 0.05% (v/v) trifluoroacetic acid in acetonitrile. Asterisk-labelled peaks within the non-reduced chromatogram represent those altered by DTT reduction.
B. Preparative scale RP-HPLC purification of peptides from the tryptic digest of Pf332 DBL domain. A total of 125 μg of digest was fractionated by elution from a Vydac C18 column (4.6 mm inner diameter × 250 mm) column using the elution conditions described in (A). Peptide fractions found to contain disulphide-bonded peptides by Edman degradation and mass spectrometry are indicated by peak numbers and correspond to those given in Table 1 (see Experimental procedures for further details).
