Fig. 3.
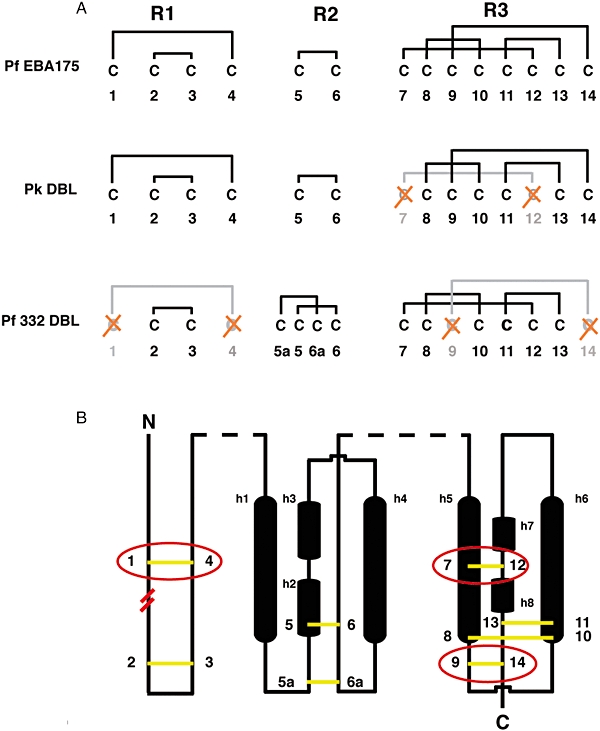
Analysis of the disulphide bond patterns within known DBL domains.
A. Disulphide-bond linkage patterns for Pf EBA-175 F2, Pk DBP and Pf332 DBL domains. Cysteine residues are numbered relative to their occurrence in the protein sequence and are clustered by their location in either subregion 1, 2 or 3 (R1, R2, R3) in the DBL domain. Crossed (red) Cys residues have been lost from the sequence and result in the subsequent loss of the corresponding disulphide bond from the protein structure, relative to the EBA-175 F2 DBL domain.
B. A two-dimensional schematic representation of the Pf EBA-175 F2 DBL domain showing the relative position of disulphide bonds (yellow) in Pf332 and Pk DBP DBL domains. Those disulphides lost from either structure are circled in red. The start of the Pf332 DBL sequence relative to the two other DBL domains is indicated by parallel red lines between the Cys-1 and Cys-2 residues. Structurally relevant helices are represented as black cylinders and are labelled h1–h8. Dashed lines (black) represent regions where sequence has not been considered.
For the Pf332 DBL domain C2 = Cys-15, C3 = Cys-21, C5a = Cys-69, C5 = Cys-73, C6a = Cys-140, C6 = Cys-144, C7 = Cys-168, C8 = 181, C10 = 187, C11 = Cys-191, C12 = Cys-235 and C13 = Cys-254.
