Fig. 4.
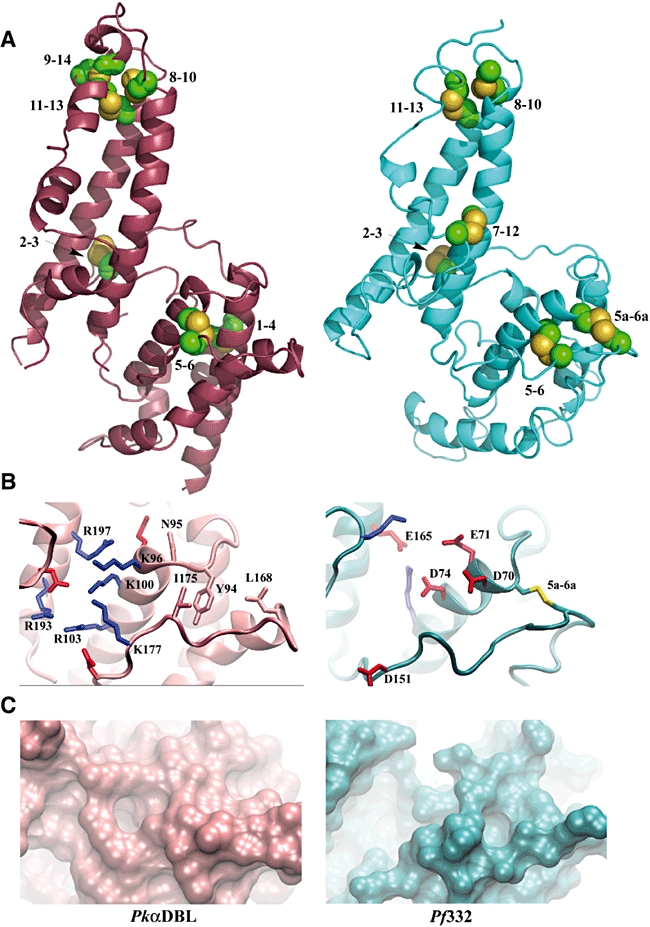
Analysis of the structure for the Pf332 DBL domain.
A. Schematic representations of the structures for the DBL domains of Pkα-DBL (magenta) and that modelled for Pf332 (blue). The disulphide-bond pattern, determined via tryptic digestion of the refolded protein and from modelling the structure of Pf332 DBL domain, is compared with that found in the structure of Pkα-DBL (PDB 2C6J). Cysteine residues are numbered and displayed in yellow.
B. A comparison of the DARC binding site from Pkα-DBL and the equivalent region in Pf332. Basic residues are shown in blue and acidic residues are shown in red. The four non-polar residues considered important for binding a sulphated tyrosine on the DARC receptor (Y94, N95, L168 and I175) are shown in the colour of the Pkα-DBL backbone. The Cys-5a–Cys-6a disulphide bond is shown in yellow.
C. The surface topographies resulting from the differences in amino acid composition in the DARC binding site for Pkα-DBL domain and the equivalent region in the Pf332 DBL domain.
