Fig. 7.
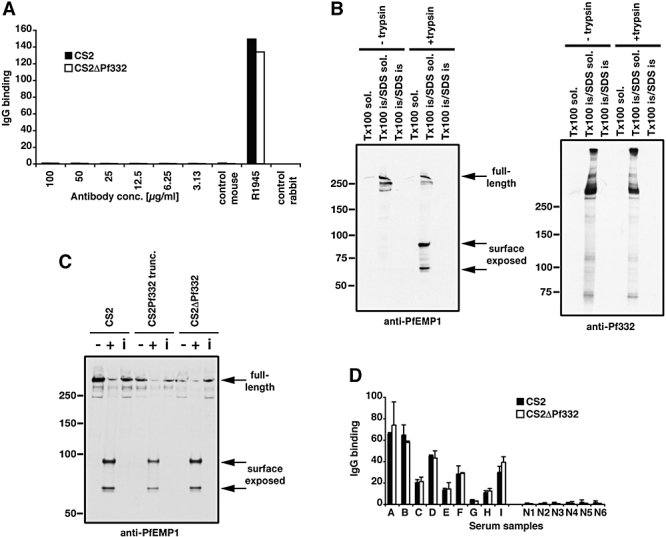
Pf332 is not located on the surface of P. falciparum-infected erythrocytes and not required for surface localization of PfEMP1.
A. FACS analysis of P. falciparum-infected trophozoites to test for the presence of Pf332 on the surface with increasing amounts of anti-Pf332 antibodies. The control sera R1945 was made by repeated injection of live CS2 parasites into rabbits.
B. Trypsin treatment of CS2-infected erythrocytes to determine surface localization for Pf332 compared with PfEMP1. The first panel contains parasites either treated or not treated with trypsin and differentially solubilized to show that PfEMP1 is sensitive to trypsin and contained within the Triton X-100-insoluble/SDS-soluble fraction. The second panel shows the same samples probed with anti-Pf332 antibodies to show that the protein is resistant to trypsin in intact cells and that it is also within the Triton X-100-insoluble/SDS-soluble fraction.
C. Trypsin treatment of intact erythrocytes infected with CS2, CS2Pf332trunc and CS2ΔPf332 to determine the presence of PfEMP1 on the surface of the host erythrocyte. For each cell line three lanes are shown: treatment without (−) and with (+) trypsin and treatment with trypsin in the presence of trypsin inhibitor (i). Full-length PfEMP1 and its cytoplasmic tail were detected using antibodies to the cytoplasmic acidic terminal segment (ATS). Full-length PfEMP1 was detected as a > 300 kDa band. Surface exposed PfEMP1 was cleaved by trypsin and results in the detection of bands between 70 and 90 kDa.
D. IgG binding to the surface of CS2- and CS2ΔPf332-infected erythrocytes using sera from malaria-exposed multigravid women from Papua New Guinea (samples A–I) compared with sera from Melbourne residents as non-exposed controls (Samples N1–5). Values are means (+SEM) from three experiments in duplicate.
