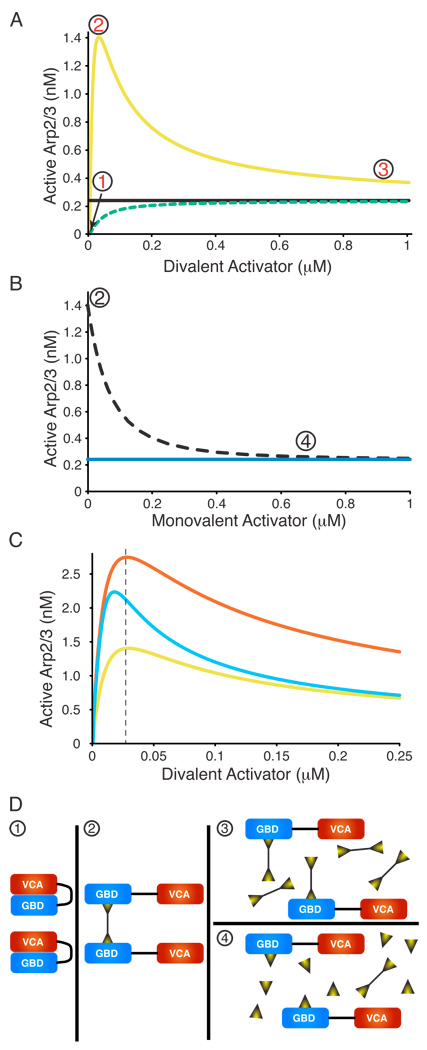
Figure 3. Modeling Predicts Unusual Behavior in Dimerizer Titrations.
The concentration of active Arp2/3 complex was modeled as a function of dimerizing WASP ligands with different properties. Here, ligand binding is concomitant with allosteric relief of autoinhibition. See Figure S9 for ligands binding to constitutively active WASP ligands. (A) Simulated titrations of monomeric WASP ligand (dashed green, e.g. EspFu 1R) or dimeric WASP ligand (yellow, e.g. EspFu 2R) into WASP plus Arp2/3 complex. Black line indicates activity of VCA. (B) Simulated titration of monomeric WASP ligand (dotted black) into WASP hyperactivated by dimeric WASP ligand (maximum activity in A). Blue line indicates activity of VCA. (C) Simulated titrations of different dimerizing ligands into WASP plus Arp2/3 complex. Titration from (A) is yellow (note change of X- and Y-scales). Sky blue curve models a dimerizer with 3-fold tighter affinity for WASP than in (A), red curve models a dimerizer:(WASP)2 complex with three-fold tighter affinity for Arp2/3 than in (A). Modeling based on 25 nM WASP, 10 nM Arp2/3 complex throughout, and the following affinities in (A) and (B)—ligand:WASP, 30 nM; active monomeric WASP:Arp2/3, 1 µM; active dimeric WASP:Arp2/3, 10 nM. (D) Cartoons illustrating the relevant species during the activating dimerizer titrations. Circled numbers correspond to the states shown in (A) and (B).