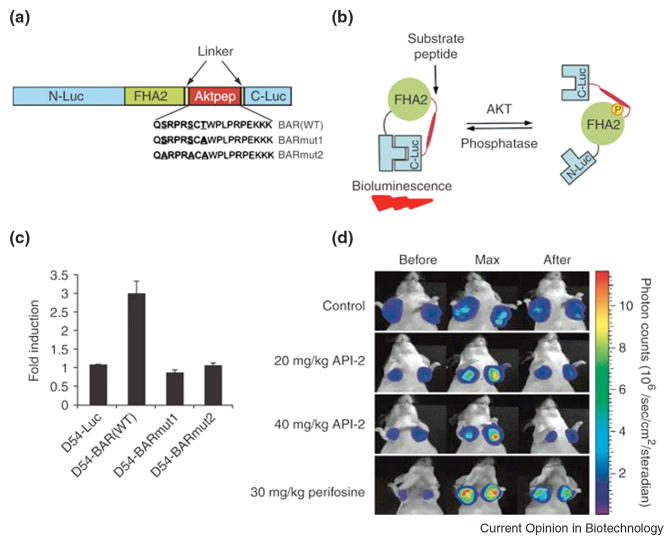
Figure 1.
Bioluminescence imaging of Akt kinase activity in vivo. (A) Domain structure of the single-chain bioluminescence Akt kinase reporter (BAR). Three versions of the reporter were developed: the wild-type (BARWT) molecule, which contains the wild-type Aktpep sequence; the BARmut1 molecule, which contains a threonine-to-alanine substitution at the primary phosphorylation site; and the BARmut2 molecule, which has all serine and threonine residues in the substrate sequence mutated to alanine. (B) The proposed mechanism of action for the BAR reporter involves Akt-dependent phosphorylation of the Aktpep domain (thick line), which results in its interaction with the FHA2 domain (right). In this form, the reporter is constrained and has minimal bioluminescence activity. In the absence of Akt activity, the N-Luc and C-Luc domains complement each other, restoring bioluminescence activity (left). (C) Stable cell lines expressing each of the reporters were treated with API-2 (40 μM), an Akt inhibitor, for 1 h. The change in bioluminescence activity compared with pretreatment values was plotted as fold induction. Data were derived from a minimum of five experiments. (D) Molecular imaging of Akt activity. Tumor-bearing mice were treated with vehicle control (20% DMSO in PBS), API-2 (20 mg/kg or 40 mg/kg) or perifosine (30 mg/kg), a phosphoinositide 3-kinase inhibitor. Images of representative mice are shown before treatment, during maximal luciferase signal upon treatment (Max), and after treatment. Adapted by permission from Macmillan Publishers Ltd: Zhang et.al., Nature Medicine, 13:1114-1119, copyright 2007.