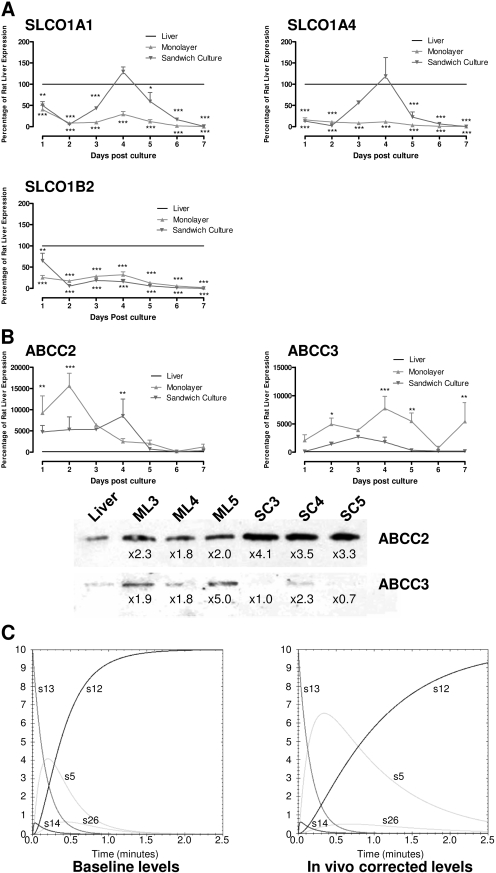
Fig. 4.
Variation in drug transporter expression during time in culture affects in vitro-in vivo correlation. Rat hepatocytes were cultured for 7 days (where cells were plated on day 0) in either monolayer (▾) or sandwich culture (▴) conformation. Samples of cells were taken each day for analysis by reverse transcriptase-PCR of the expression of the influx transporters SLCO1A1, 1A4, and 1B2 (A) and the efflux transporters ABCC2 and C3 (B). Data are expressed as a percentage of the level in rat liver (100%, —), with RNA isolated from the fresh rat livers subsequently used to prepare hepatocytes cultures. Data were analyzed by two-way analysis of variance with Bonferroni post hoc test and show where expression in hepatocytes is significantly different from liver expression (***, p < 0.001; n = 3; error bars are S.E.M.; where no error bars are observed, they are contained within the limits of the data point). Protein levels of ABCC2 and C3 were also measured by Western blot after 3, 4, and 5 days of culture and compared with the level from fresh rat livers subsequently used to prepare hepatocytes cultures (B). Finally, in silico modeling was used to compare how altered protein levels of ABCC2 and C3 might affect disposition of CDFDA/CDF in 4-day sandwich cultures (baseline levels) versus in vivo-corrected levels (C). Kinetic parameters for each of the major CDFDA/CDF species are shown and map to those shown in Fig. 1.