Fig. 7.
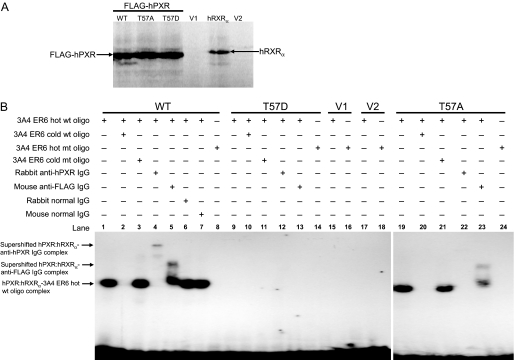
Phosphomimetic mutation at Thr57 impairs DNA binding of hPXR. A, in vitro synthesis of proteins for wild-type and mutant FLAG-hPXR, as well as for hRXRα, as described under Materials and Methods. Equal amounts of protein were observed from 2-μl in vitro transcription and translation reactions for wild-type (WT), alanine mutant (T57A), and aspartate mutant (T57D) hPXRs. No specific proteins were observed from the negative control reactions using the vectors, pcDNA3-FLAG (V1) and pSG5 (V2), for hPXR and hRXRα, respectively. B, electrophoretic mobility shift assay was performed with in vitro-translated FLAG-hPXR, FLAG-hPXRT57D, or FLAG-hPXRT57A and hRXRα proteins, as well as 32P-labeled CYP3A4 PXR DNA binding sequence (indicated as 3A4ER6) as described under Materials and Methods. Equal amount of hRXRα was added to all the reactions and was not shown in the figure. FLAG-hPXR and FLAG-hPXRT57A bound to and formed a complex with hot wild-type (wt) oligo, and this complex (lanes 1 and 19) was efficiently competed with by cold wt oligo (lanes 2 and 20) but not by cold mutant (mt) oligo (lanes 3 and 21) at 500 M excess. Addition of 2 μg of rabbit anti-hPXR IgG supershifted the FLAG-hPXR-hot wt oligo complex (lane 4) and vanished the FLAG-hPXRT57A-hot wt oligo complex (lane 22) (Saradhi et al., 2005). Addition of 2 μg of mouse anti-FLAG IgG supershifted both the FLAG-hPXR-hot wt oligo complex (lane 5) and the FLAG-hPXRT57A-hot wt oligo complex (lane 23). Addition of 2 μg of rabbit (lane 6) and mouse (lane 7) normal IgG failed to supershift or vanish the FLAG-hPXR-hot wt oligo complex. No complex was detected for FLAG-hPXR (lane 8) and FLAG-hPXRT57A (lane 24) with hot mt oligo. No complex was detected with phosphomimetic mutant FLAG-hPXRT57D (lanes 9–14) and vectors, pcDNA3-FLAG (V1) (lanes 15 and 16), and pSG5 (V2) (lanes 17 and 18). Data shown are from a representative experiment.