Abstract
Phencyclidine (PCP) is a mechanism-based inactivator of cytochrome P450 (P450) 2B6. We have analyzed several steps in the P450 catalytic cycle to determine the mechanism of inactivation of P450 2B6 by PCP. Spectral binding studies show that binding of benzphetamine, a type I ligand, to P450 2B6 was significantly affected as a result of the inactivation, whereas binding of the inhibitor n-octylamine, a type II ligand, was not compromised. Binding of these ligands to P450 2B6 occurs in two phases. Stopped-flow spectral analysis of the binding kinetics of benzphetamine to PCP-inactivated 2B6 revealed a 15-fold decrease in the rate of binding during the second phase of the kinetics (k1 = 5.0 s–1, A1 = 30%; k2 = 0.02 s–1, A2 = 70%, where A2 indicates the fractional magnitude of the second phase) compared with the native enzyme (k1 = 8.0 s–1, A1 = 58%; k2 = 0.3 s–1, A2 = 42%). Analysis of benzphetamine metabolism by the inactivated protein using liquid chromatography/electrospray ionization/mass spectrometry showed that the rates of formation of nor-benzphetamine and hydroxylated nor-benzphetamine were decreased by 75 and 69%, respectively, whereas the rates of formation for amphetamine, hydroxybenzphetamine, and methamphetamine showed slight but statistically insignificant decreases after the inactivation. The rate of reduction of P450 2B6 by NADPH and reductase was decreased by 6-fold as a result of the modification by PCP. In addition, the extent of uncoupling of NADPH oxidation from product formation, a process leading to futile production of H2O2, increased significantly during the metabolism of ethylbenzene as a result of the inactivation.
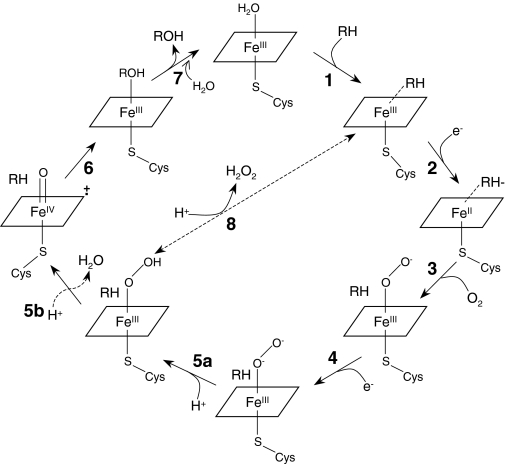
Cytochrome P450 (P450) 2B6 has been shown to be inactivated by phencyclidine (PCP) in a mechanism-based manner (Jushchyshyn et al., 2003). Mechanism-based inactivators of P450s cause covalent modification of the active site that compromises one or more steps in the catalytic cycle of the enzyme, thereby rendering the enzyme inactive (Hollenberg et al., 2008). The catalytic mechanism of P450s involves an overall two-electron reduction of O2 to form a reactive oxygenating intermediate(s). As shown in Fig. 1, the first step during catalysis involves binding of substrate to the P450 that is in the resting ferric state [1], which stimulates the second step involving a one-electron transfer from NADPH-P450 reductase (reductase) to the P450 [2]. The subsequent steps are binding of molecular oxygen to the heme iron [3], transfer of a second electron via either the reductase or cytochrome b5 [4], formation of a reactive iron-oxygen species [5], insertion of oxygen into the substrate [6], and finally release of the oxygenated product with the return of the P450 to the resting ferric state [7]. Uncoupling of the catalytic cycle could occur at step [5a], where dissociation of the hydroperoxide ligand results in conversion of the hydroperoxo intermediate to the ferric form.
Fig. 1.
Individual steps that comprise the catalytic cycle of P450. RH, substrate; H2O2, hydrogen peroxide; ROH, hydroxylated product; Cys, the conserved axial cysteine ligand.
The inactivation of P450 by mechanism-based inactivators has been suggested to occur by at least three distinct mechanisms. These include alkylation of the heme moiety, covalent binding of a reactive intermediate to the apoprotein, or cross-linking of the heme to the apoprotein. These general structural effects are responsible for modification of one or more of the individual steps in the catalytic cycle that lead to the irreversible changes in the activity of the P450.
Mechanism-based inactivators have been used as tools to identify key residues in the active site of P450s that are involved in substrate binding and/or catalysis. Acetylenic compounds such as 9-ethynylphenanthrene have been shown to inactivate P450 2B1 by binding to the apoprotein (Roberts et al., 1995a). Further studies showed that the 9-ethynylphenanthrene-modified enzyme was impaired in its ability to undergo reduction by reductase and NADPH (step 2) and that oxygen consumption was more uncoupled from product formation, resulting in increased formation of hydrogen peroxide compared with native enzyme (Roberts et al., 1995b). Chloramphenicol, another mechanism-based inactivator of P450 2B1, caused covalent modification of a lysine residue in the active site, and the loss in catalytic activity was the result of inhibition of the transfer of electrons from reductase to P450 (Halpert et al., 1985). Covalent modification of the P450 2B1 apoprotein by N-benzyl-1-aminobenzotriazole had no effect on the substrate binding step; however, the modification caused a decrease in the rate of reduction and changes in the orientation of substrates in the active site, as indicated by a different product distribution from metabolism of benzphetamine compared with native enzyme (Kent et al., 2004).
In our studies with P450 2B6 and PCP, only ∼60 to 80% loss in enzymatic activity for the metabolism of 7-ethoxy-4-trifluoromethylcoumarin (7-EFC) is observed, suggesting that the PCP-modified P450 is capable of carrying out catalysis, but at a much slower rate than the unmodified protein. To determine the mechanism by which PCP causes the loss in 2B6 activity, we investigated the effects of inactivation by PCP on several of the individual steps of the catalytic cycle. These steps include substrate binding, donation of the first electron, and uncoupling of electron transfer from NADPH to product formation, as well as substrate hydroxylation. It is important to analyze each individual step because the inactivation of P450 2B6 by PCP may lead to modification of the rate-limiting step in the reaction cycle or the modification of several steps in the overall cycle that could collectively compromise the overall rate of catalysis.
Materials and Methods
Materials. PCP hydrochloride, NADPH, glutathione, bovine serum albumin, catalase, benzphetamine hydrochloride, n-octylamine, ethylbenzene (EB), and its metabolite 1-phenylethanol were purchased from Sigma-Aldrich (St. Louis, MO). Trifluoroacetic acid was purchased from Pierce Chemicals (Rockford, IL). 7-EFC was from Invitrogen (Carlsbad, CA). 10-YM (10,000 molecular weight cutoff) Centricon microconcentrators were from Millipore Corporation (Billerica, MA).
Reconstitution, Inactivation, and Activity Assays. P450 2B6 (1 μM) was reconstituted with reductase (2 μM) for 45 min at 4°C, at which time catalase (100 units) and 50 mM potassium phosphate (KPi) buffer, pH 7.4, were added to give a final volume of 2 ml. This primary reaction mixture was divided into two samples of equal volume. The inactivation involved incubating one sample with 100 μM PCP and 1 mM NADPH, whereas the other sample received an equal volume of water (53 μl), and the reactions were incubated at 30°C for 20 min. After 0, 10, and 20 min of incubation, aliquots (10 pmol of P450) of the primary reaction mixtures were transferred to 990 μl of a secondary reaction mixture containing 7-EFC (100 μM, much greater than the reported Km) (Code et al., 1997; Hanna et al., 2000), NADPH (0.2 mM), and bovine serum albumin (40 μg/ml) in 50 mM KPi, pH 7.4, and incubated at 30°C for 5 min. The enzyme activity was quenched by the addition of ice-cold acetonitrile (final concentration of 25%). The 7-EFC O-deethylation activity was measured spectrofluorometrically as described previously (Buters et al., 1993). The fluorescence of the O-dealkylated product was measured at room temperature on an RF5301 spectrofluorometer (Shimadzu, Kyoto, Japan) with excitation at 410 nm and emission at 510 nm.
Determination of Spectral Dissociation Constants. Control and inactivated samples were dialyzed separately for 4 h at 4°C against 1 liter of 100 mM KPi buffer, pH 7.4, containing 20% glycerol, and this was followed by a second dialysis overnight with fresh buffer. The control and inactivated samples were diluted to the same volume (1 ml) using fresh dialysis buffer. A 100-μl aliquot (100 pmol) of each sample was removed and assayed for activity using 7-EFC as a probe substrate. Another aliquot (100 μl) was removed to measure the P450 concentration using the reduced-CO difference spectrum. Equal volumes of control or inactivated P450 2B6 were placed into sample and reference cuvettes. n-Octylamine in dimethyl sulfoxide (DMSO; 0.5–60 μM) was added to the sample cuvette, whereas the reference cuvette received equal volumes of DMSO, and the difference spectra between the sample and reference cuvettes were recorded by scanning from 350 to 500 nm on a UV-visible spectrophotometer (Shimadzu, UV-250 1 PC, IL). Similar experiments were performed using benzphetamine in water (10–200 μM), where the reference cuvette received water instead of DMSO. The inverse of the changes in absorbance measured at 390 and 419 nm (benzphetamine) and at 425 and 390 nm (n-octylamine) was plotted as a function of the inverse of the concentrations of each compound to determine the spectral binding constant (Ks) value (determined from the reciprocal of the negative intercept with the x-axis). The Ks is defined as the concentration of the compound that results in 50% of the theoretical maximal spectral change (Estabrook and Werringloer, 1978).
Kinetics of Benzphetamine Binding. P450 2B6 (10 μM) was reconstituted with reductase (20 μM), and inactivation by PCP was performed as described above. The samples were concentrated using 10-YM (10,000 molecular weight cutoff) Centricon microconcentrators (Millipore Corporation), washed three times with 500 μl of 50 mM KPi buffer, pH 7.4, and resuspended in 4 ml of 50 mM KPi buffer, pH 7.4. An aliquot (30 μl) was removed from each sample to measure the P450 concentration using the reduced-CO difference spectrum to normalize the samples for the amount of protein present. Fresh buffer solutions consisting of 50 mM KPi, pH 7.4, with or without 1 mM benzphetamine were prepared. The kinetic studies were performed using a double-mixing Hi-Tech Scientific (Salisbury, Wiltshire, UK) model SF-61 DX2 stopped-flow spectrophotometer controlled by KinetAsyst (version 3.16; Hi-Tech Scientific). One syringe was filled with the sample, whereas the second syringe was filled with the buffer solution. Three or more shots were performed to flush the stopped-flow lines and to obtain a baseline. After the flushing was complete, the buffer in the second syringe was replaced with the benzphetamine-containing buffer solution (1 mM), and several shots were performed and scanned for 100 s each. The kinetics data were analyzed using a parallel fitting routine with two irreversible exponential steps (Yanev et al., 2000). The equation used for the calculations was A = A1(1 – ek t1) + A2(1 – ek t2) + C. The rates of the spectral changes at 390 nm were obtained from the best fits with the smallest residuals.
Liquid Chromatography/Mass Spectrometry Analysis of Benzphetamine Metabolism by P450 2B6. P450 2B6 (1 μM) was reconstituted with reductase (2 μM) and inactivated as described above. The samples were then prepared for further analysis as described previously (Kent et al., 2004). After the concentration and washing of the control and inactivated samples, benzphetamine was added to each sample (500 μM final concentration), and metabolism was initiated by the addition of 1 mM NADPH to the control and inactivated samples. The reactions were terminated by adding 500 μl of 8% bicarbonate buffer, pH 10, followed by adding the internal standard d3-norbenzphetamine (2.2 nmol). The metabolites were extracted twice with ethyl acetate; the organic phases were pooled; and a small amount (50 μl) of acetic acid was added. The samples were dried under a steady stream of nitrogen gas, and the residues were redissolved in 200 μl of 50% acetonitrile. Aliquots of 50 μl of each sample were then injected onto a C8 reverse-phase column (Zorbax 4.6 × 250 mm, 5 μm; Agilent Technologies, Palo Alto, CA) that was developed with a linear gradient of 30 to 90% B (solvent A: water, 0.1% formic acid; solvent B: CH3CN, 0.1% formic acid) over 7 min with a flow rate of 0.3 ml/min. The operating conditions for the LCQ Classic (Thermo Fisher Scientific, Waltham, MA) mass spectrometer with an electrospray ionization interface were as follows: positive ion mode with data-dependent scanning, sheath gas and auxiliary gas at 100 and 40 arbitrary units, respectively; spray voltage at 3 kV; capillary temperature at 230°C; and capillary voltage at 3.5 V. The mass spectrometer and all the peripheral components were controlled by the Xcalibur software (Thermo Fisher Scientific).
Anaerobic Reduction of Native and PCP-Inactivated P450 2B6. The samples were prepared as described previously for the stopped-flow studies. To obtain anaerobic conditions, the experiment was carried out as described previously (Roberts et al., 1995a). In brief, the sample (native or inactivated P450) was placed in a tonometer with 3 units of protocatechuic acid dioxygenase and 50 μM protocatechuic acid (PCA) in the side arm as an oxygen scavenging system. Anaerobic conditions were achieved by several cycles of alternating argon gas containing ≤0.5 ppm O2 and vacuum over a period of 20 to 30 min, followed by the addition of PCA from the side arm of the tonometer. A second tonometer contained the buffer and 50 μM PCA only or buffer with 50 μM PCA, 1 mM NADPH, and 1 mM benzphetamine. Substrate binding to the ferric P450 increases the rate of reduction of P450 (Backes and Eyer, 1989; Eyer and Backes, 1992); therefore, benzphetamine was included in the buffers to achieve the maximal rates of reduction. The NADPH samples were bubbled with CO, and 3 units of protocatechuic acid dioxygenase were added to achieve anaerobic conditions. To flush the lines and obtain a stable baseline, the second tonometer containing only anaerobic buffer was used, and several shots were performed. Equal volumes of both reactants were mixed in the stopped-flow spectrophotometer and monitored using diode array detection. Three or more shots were performed using a tonometer with buffer containing both NADPH and benzphetamine, and the formation of the reduced P450-CO complex was monitored at 450 nm. The data were recorded at 25°C with scanning at 200 scans/s and a run time of 150 s. The kinetic data were analyzed using a parallel exponential fitting routine, and the best fits with the smallest residuals were obtained when two exponential steps were used.
Stoichiometry of NADPH Oxidation, EB Hydroxylation, and H2O2 Formation. P450 2B6 (4 μM) was reconstituted with reductase (8 μM) for 45 min on ice. PCP (200 μM) and 50 mM KPi buffer, pH 7.4, were added to give a total volume of 2.6 ml. The reconstituted mixture was divided into two samples of equal volume (1.3 ml). The reconstituted proteins were pre-equilibrated at 30°C for 5 min. NADPH (1 mM) was added to one of the sample tubes to initiate the reaction, whereas the other sample received an equal volume of water (68 μl). After 30 min of incubation, the samples were placed on ice and transferred to YM-10 Centricon microconcentrators. Washing and concentration were performed as described above. The proteins were resuspended in 1.3 ml of 50 mM KPi buffer, pH 7.4, and were prepared for further analysis. An aliquot (62 μl) of each sample was removed and placed in a new tube on ice for recording the absolute and reduced-CO spectra to determine the amount of P450 recovered. All of the samples were normalized for the amount of protein recovered. For the analysis of NADPH oxidation activity, an aliquot of the reconstituted system containing 160 pmol of 2B6 and 0.2 mM NADPH was diluted with 50 mM KPi buffer, pH 7.4, to a total volume of 0.5 ml and placed in a cuvette at 30°C in the UV-visible spectrophotometer (Shimadzu, UV-250 1PC) connected to a temperature controller. The change in absorbance was monitored at 340 nm for 2 min after the addition of 1 μl of methanol or EB (250 mM) to initiate the reaction. An extinction coefficient of 6.22 mM–1 cm–1 for the difference between NADPH and NADP+ at 340 nm (Gorsky et al., 1984) was used to calculate the amount of NADPH oxidized in the assay. EB, when present, was added to a final concentration of 500 μM. The reaction was terminated after 2 min by the addition of 250 μl of 72% trichloroacetic acid, and the samples were placed on ice for 20 min. The samples were centrifuged at maximum speed for 20 min at 4°C in a microcentrifuge to remove the precipitated protein before further analysis. An aliquot (0.6 ml) was removed for the determination of H2O2 formation spectrophotometrically using the ferrithiocyanate method by measuring the OD at 480 nm (Hildebrandt et al., 1978). In brief, a fresh solution of 10 mM ferrous ammonium sulfate and 2.5 mM potassium thiocyanate was prepared during the centrifugation step. An aliquot (0.6 ml) of each sample was mixed with 0.6 ml of the ferrithiocyanate solution to allow the interaction with H2O2 to form a complex that exhibits an absorption maximum at 480 nm.
For analysis of the amount of EB hydroxylated to 1-phenylethanol, the reaction mixture contained 160 pmol of P450, and samples were incubated either in the presence or absence of 500 μM EB in a total volume of 0.5 ml. Reactions were initiated by the addition of NADPH (1 μM final), incubated at 30°C for 2 min, and quenched with 50 μl of 3 M H2SO4. Each reaction was centrifuged for 20 min at maximum speed, and the supernatant was carefully removed. An aliquot (190 μl) of the supernatant was used for high-performance liquid chromatography analysis of the EB hydroxylation as described by Sams et al. (2004). Each reaction was carried out in triplicate, and the rates of NADPH oxidation, formation of 1-phenylethanol, and H2O2 production were expressed as nanomole of product per minute per nanomole of P450.
Results
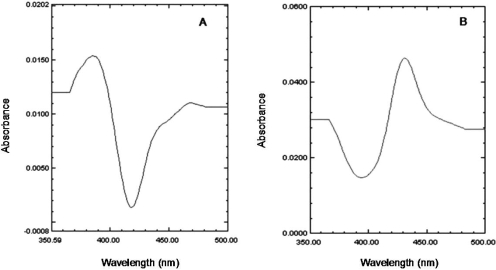
Effect of Inactivation of P450 2B6 by PCP on Substrate Binding. To determine the ability of P450 2B6 to bind other substrates after inactivation by PCP, we performed binding studies using the substrate benzphetamine and the inhibitor n-octylamine. The dissociation constants (Ks) for the binding of benzphetamine and n-octylamine were determined spectrally as described under Materials and Methods. As shown in Fig. 2, binding of benzphetamine produced a typical type I binding spectrum with a maximum absorbance peak appearing at 390 nm and a trough at 419 nm. The low molecular weight inhibitor n-octylamine, which coordinates with the heme iron, produced the expected type II difference spectrum, with a minimum at 390 nm and a maximum absorption peak at 425 nm (Fig. 2). The resulting changes in absorbance with increasing concentrations of benzphetamine or n-octylamine were plotted as the inverse of the change in absorbance versus the inverse of the benzphetamine or n-octylamine concentrations. From these plots, the Ks values were determined. Table 1 shows the Ks values of both compounds obtained with control samples that were incubated with PCP in the absence of NADPH versus the PCP-inactivated samples. These results suggest that the inactivation of P450 2B6 by PCP did not affect the binding affinity of the low molecular weight inhibitor n-octylamine to the enzyme; however, the bulkier substrate benzphetamine displayed a significant decrease in its affinity for P450 2B6 as indicated by the 7-fold increase in the Ks.
Fig. 2.
Difference spectra for the binding of benzphetamine and n-octylamine to P450 2B6. The addition of benzphetamine (200 μM) results in a typical type I difference spectrum (A), whereas addition of n-octylamine (60 μM) produced the classic type II spectrum (B) with the P450 2B6. For benzphetamine, the changes in the difference between maximum absorbance at 390 nm and the minimum at 419 nm were used to calculate the spectral dissociation constant, Ks. The difference between the maximum at 425 nm and the trough at 390 nm was used to calculate the Ks for n-octylamine. In both cases, a plot was generated using the inverse of the change in absorbance versus the inverse of the increasing concentrations of the compound to determine the Ks.
TABLE 1.
Dissociation constants for the binding of type I and type II compounds to unmodified and PCP-inactivated P450s 2B6
|
P450 2B6
|
Ksa
|
|
|---|---|---|
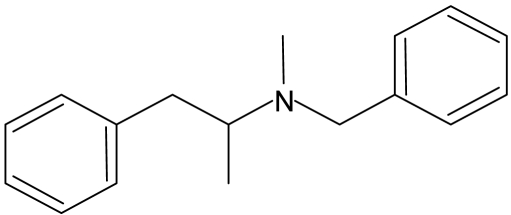 Benzphetamine (type
I) Benzphetamine (type
I)
|
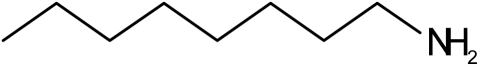 n-Octylamine (type II) n-Octylamine (type II)
|
|
| μM | μM | |
| Native | 36 ± 1 | 9 ± 5 |
| Inactivated | 258 ± 12 | 8 ± 6 |
Binding spectra were determined as described under Materials and Methods. The dissociation constants (Ks) were determined as described in the text. The values represent the means and S.D. from three independent experiments.
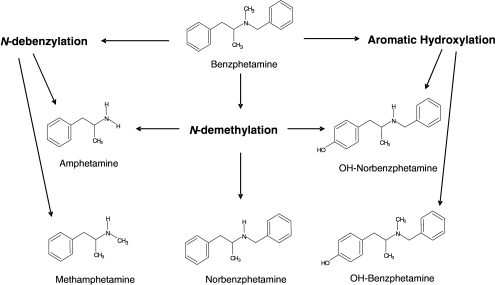
Metabolism of Benzphetamine by P450 2B6. The spectral binding data indicated that benzphetamine was able to bind in the active site of PCP-inactivated 2B6 but with a lower affinity. This suggested that the binding is sterically hindered by the presence of covalently bound PCP or that the orientation of benzphetamine in the active site is different because of the presence of the covalently bound PCP. To address these possibilities, we analyzed the metabolism of benzphetamine by 2B6 using methods previously published (Kent et al., 2004) to determine whether inactivation by PCP had affected the ability of the enzyme to produce the full spectrum of metabolites. The data from these experiments might provide information regarding possible changes in the binding orientation of benzphetamine in the active site. The pathways for the metabolism of benzphetamine by P450s 2B1 (Fig. 3) and 2B4 have been reported (Gruenke et al., 1995; Kent et al., 2004). However, the metabolism of benzphetamine by human P450 2B6 has only been characterized previously by measuring the formation of formaldehyde generated by the N-demethylation pathway (Reed and Hollenberg, 2003). Liquid chromatography/mass spectrometry analyses of benzphetamine metabolism by human P450 2B6 and PCP-inactivated 2B6 were performed as described under Materials and Methods. Table 2 summarizes the results for metabolite semiquantitation from the incubations with native and PCP-inactivated P450 2B6. The overall rate of benzphetamine metabolism by the inactivated 2B6 appeared to be reduced by approximately 50%. Because only one authentic metabolite was available to be used as an internal standard for this quantitation approach, it is possible that each metabolite may have a different ionization response in the mass spectrometer, which only allows for an estimation of ratios rather than accurate quantitation for all the metabolites. The largest differences were seen in the rates for the formation of norbenzphetamine and hydroxylated norbenzphetamine by the inactivated sample, where 75 and 69% decreases were observed, respectively. These results suggest that one or more pathways in the metabolism of benzphetamine were significantly affected by PCP inactivation of 2B6 (refer to Fig. 3), presumably because of changes in the binding orientation of benzphetamine in the active site of the PCP-inactivated enzyme.
Fig. 3.
Pathways for the metabolism of benzphetamine by P450 2B enzymes.
TABLE 2.
LC/MS analysis of benzphetamine metabolism by P450 2B6
The incubations, metabolite extraction, and analysis by LC/MS were described under Materials and Methods. Quantitation was performed in the presence of an internal standard (d3-norbenzphetamine), and the results were normalized to the control (native) sample as 100%.
|
Metabolite
|
Area under the Peak
|
Percentage of Native
|
|
|---|---|---|---|
| Native | Inactivated | ||
| OH-benzphetamine | 79 ± 6 | 57 ± 17 | 72 |
| OH-norbenzphetamine | 7.0 ± 2 | 2.2 ± 1 | 31* |
| Norbenzphetamine | 63 ± 0.7 | 16 ± 10 | 25* |
| Methamphetamine | 2.3 ± 2 | 1.7 ± 1 | 74 |
| Amphetamine | 1.2 ± 1 | 0.7 ± 0.5 | 58 |
, indicates statistical significance; P < 0.05.
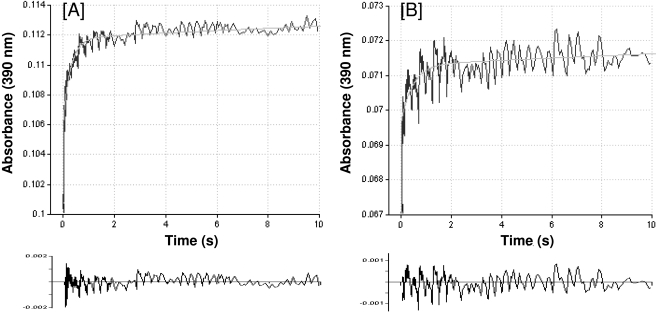
Rates of Benzphetamine Binding. The data so far describe the effects of inactivation of 2B6 by PCP on the initial substrate binding step of the overall P450 catalytic cycle. These results suggest that the inactivation leads to a decrease in the binding affinity of benzphetamine and a concomitant change in its binding orientation in the catalytic site. To increase our understanding of the observed effects, we considered the possibility that the inactivation leads to a decrease in the initial rate of substrate binding, which may be responsible for the observed decreases in binding affinity and/or rates of metabolism. To address this hypothesis, we performed stopped-flow experiments to determine the kinetics of benzphetamine binding to the native and PCP-inactivated proteins. As shown in Fig. 4, the binding of benzphetamine to the native or PCP-inactivated 2B6 is biphasic. In the control sample, the first phase is relatively fast (k1 = 8.0 s–1, A1 = 58%) compared with the second phase, which is much slower (k2 = 0.3 s–1, A2 = 42%). Qualitatively similar results were seen with the inactivated sample in the first phase (k1 = 5.0 s–1, A1 = 30%). However, the rate was reduced by 38%, and the amount of binding in that phase was decreased by 28% compared with the control sample. In addition, the second phase was markedly slower than that of the native enzyme with the rate decreased by 15-fold (k2 = 0.02 s–1, A2 = 70%). Table 3 shows the overall rates for benzphetamine binding to both proteins. For easier comparison the results were expressed as percentage of control. The data show that the inactivation of 2B6 by PCP led to an overall 70% decrease in the rate of benzphetamine binding, which may partially explain the decrease in the binding affinity and the rate of metabolism of benzphetamine.
Fig. 4.
Time course for the binding of benzphetamine to P450 2B6. P450 2B6 was incubated in the absence or presence of PCP, and the reaction mixtures were subsequently treated as described under Materials and Methods. Kinetic traces were acquired at 390 nm over time, and the rate of ΔA390 for benzphetamine binding to native P450 2B6 (A) and PCP-inactivated 2B6 (B) was fitted to biexponential plots. Analysis of residuals for the biexponential fits is shown at the bottom of each panel. The rates of the biphasic binding are presented in Table 3.
TABLE 3.
Rates of benzphetamine binding to native and PCP-inactivated P450 2B6
The inactivation of P450 2B6 and the stopped-flow studies to determine the rates of benzphetamine binding were performed as described under Materials and Methods.
|
P450 2B6
|
Phase 1
|
Phase 2
|
Σ
knb
|
Percentage of Native
|
||
|---|---|---|---|---|---|---|
| A1a | k1 | A2 | k2 | |||
| % | s-1 | % | s-1 | %n | % | |
| Native | 58 | 8.0 ± 1 | 42 | 0.3 ± 0.01 | 4.8 | 100 |
| Inactivated | 30 | 5.0 ± 0.6 | 70 | 0.02 ± 0.003 | 1.5 | 31 |
Percentage of the change in amplitude is of the total change in amplitude [An/(A1 + A2)] × 100.
Σ kn (%n) is defined as Σ [(kn)(% in phase n)] and is the summation of [(k1)(% in phase 1)] + [(k2)(% in phase 2)]. The data represent the averages and S.D. of three shots.
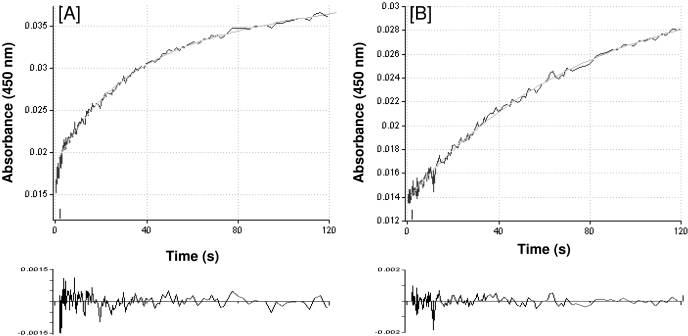
Reductase-Dependent Steps and Rates of Reduction. Mechanism-based inactivators may affect one or more steps of the P450 catalytic cycle. The first step after the binding step in the catalytic cycle is the reduction of the heme iron from the ferric to the ferrous state by the transfer of one electron from NADPH via reductase. Subsequent binding of molecular oxygen to the ferrous iron then leads to formation of the Fe2+-O complex (Fig. 1), which acts as a precursor to the activated iron oxygen species. We investigated the possibility that the inactivation of P450 2B6 by PCP may lead to modification of its reduction step. When CO is added to the ferrous enzyme, it binds very rapidly to the heme iron to form the ferrous carbonyl complex that produces the distinctive peak at ∼450 nm in the absorbance spectrum. Therefore, the reduction of P450 can be monitored in the presence of CO by following the formation of the absorbance peak at 450 nm. Thus, we can determine whether modification of P450 by PCP changes the rate of reduction by the reductase and NADPH. The anaerobic reduction of P450 2B6, as measured by its ability to form the Fe2+-CO complex, was measured using stopped-flow spectrophotometry. As shown in Fig. 5A, the reduction of native 2B6 is biphasic, comprising a fast initial phase (k1 = 0.35 s–1, A1 = 19%) and a slower second phase (k2 = 0.02 s–1, A2 = 81%). A significant difference was observed with the PCP-inactivated sample (Fig. 5B), where the fast phase was almost nonexistent and the second phase was similar to, but slightly slower than, that observed for the control sample (k2 = 0.014 s–1, A2 = 100%). Table 4 summarizes the results of these experiments, and the overall rates revealed that the inactivated 2B6 exhibited a decrease of approximately 83% in its ability to be reduced via reductase.
Fig. 5.
Time course for the anaerobic reduction of ferric P450 2B6 monitored at 450 nm. A, anaerobic reduction of native P450 2B6 is shown with the trace fitted to a biexponential plot. B, anaerobic reduction of PCP-inactivated 2B6 with a single exponential plot. Analysis of residuals for the biexponential and single exponential fits is shown at the bottom of each panel. The experiments are described under Materials and Methods, and the rates calculated from these plots are presented in Table 4.
TABLE 4.
Anaerobic reduction of native and PCP-inactivated P450 2B6 by NADPH-mediated reductase in the presence of CO
The inactivation of P450 2B6 and the stopped-flow studies to determine the rates of reduction of P450s were performed as described under Materials and Methods.
|
P450 2B6
|
Phase 1
|
Phase 2
|
Σ
knb
|
Percentage of Native
|
||
|---|---|---|---|---|---|---|
| A1a | k1 | A2 | k2 | |||
| % | s-1 | % | s-1 | %n | % | |
| Native | 19 | 0.35 | 81 | 0.020 | 8.3 | 100 |
| Inactivated | N.D. | N.D. | 100 | 0.014 | 1.4 | 17 |
Percentage of the change in amplitude is of the total change in amplitude [An/(A1 + A2)] × 100.
Σ kn (%n) is defined as Σ [(kn)(% in phase n)] and is the summation of [(k1)(% in phase 1)] + [(k2)(% in phase 2)]. The data represent the results from the averages of three shots. N.D., not detected.
Product Formation and Changes in the Extent of Uncoupling. To determine the effects of inactivation of 2B6 by PCP on the extent of uncoupling of the P450 reaction cycle, we determined the ratios of NADPH oxidation to product formation (1-phenylethanol) and H2O2 production by measuring NADPH oxidation and H2O2 production during the 1-hydroxylation of EB by the native and inactivated 2B6. With the native 2B6, 93% of the reducing equivalents used in the absence of substrate resulted in H2O2 formation (Table 5). It is possible that the rest of the reducing equivalents led to the formation of water (Gorsky et al., 1984). In the presence of the substrate, EB, the native protein exhibited a 3-fold increase in the rate of NADPH oxidation to 81 nmol/min/nmol P450, and 1-phenylethanol and H2O2 were produced at rates of 54 and 25 nmol product/min/nmol P450, respectively, giving a ratio of NADPH/1-phenylethanol/H2O2 of 1.0: 0.7:0.3 (Table 5). In the presence of EB, the inactivated enzyme showed a 27% decrease in the rate of NADPH oxidation compared with the native enzyme. The ratio of NADPH consumption to 1-phenylethanol and H2O2 formation was 1.0:0.3:0.7 for the inactivated enzyme. The decrease in the rate of formation of 1-phenylethanol of 63% corresponded well with the loss in the 7-EFC O-deethylation activity (65%). These data suggest that the inactivation of P450 2B6 by PCP in part leads to a concomitant increase in the uncoupling of the enzyme from 1-phenylethanol formation and an increase in the futile auto-oxidation pathway leading to H2O2 formation.
TABLE 5.
Effects of inactivation by PCP on the rates of NADPH oxidation, product formation, and hydrogen peroxide production by P450 2B6
The incubations, assays, and analyses are described under Materials and Methods. The values represent the mean and S.D. of three determinations done in duplicate.
| P450 2B6 | EB | NADPH | 1-PhE | H2O2 | Ratio of NADPH/1-PhE/H2O2 | Activity Remaininga |
|---|---|---|---|---|---|---|
| nmol/min/nmol P450 | nmol/min/nmol P450 | nmol/min/nmol P450 | % | |||
| Native | - | 28 ± 4 | - | 26 ± 1 | 1.0:0.0:0.9 | 100 |
| Native | + | 81 ± 2 | 54 ± 4 | 25 ± 1 | 1.0:0.7:0.3 | 100 |
| Inactivated | + | 59 ± 7 | 20 ± 4 | 46 ± 2 | 1.0:0.3:0.7 | 35 |
1-PhE, 1-phenylethanol.
The percentage activity remaining was determined from the 7-EFC O-deethylation assay as described under Materials and Methods.
Discussion
Earlier studies have shown that PCP inactivates P450 2B6 in a mechanism-based manner (Jushchyshyn et al., 2003) and that the inactivation occurs via covalent modification of the apoprotein. The reactive intermediate of PCP that reacts with the protein and is responsible for the loss in activity was trapped using both glutathione and N-acetylcysteine and was identified as 4-hydroxy-2,3-epoxy piperidine (Shebley et al., 2006). However, the mechanism(s) by which protein modification results in the inactivation has not been determined. In this study, we investigated several steps in the catalytic cycle of P450 to identify those steps in the cycle that were compromised by modification of 2B6 by PCP and led to inactivation. Substrate binding to P450s can be monitored by tracking changes in the positions and intensities of the heme Soret spectral band as an indicator of changes in the heme spin states. Although indirect, this approach is often used to determine substrate-binding affinities because of its experimental simplicity, ease of sample preparation, and reliance on equipment that is readily available in most laboratories working on P450s. Benzphetamine binds reversibly to P450 2B4 and causes spectral changes characteristic of type I compounds with a decrease in the absorbance at 420 nm and an increase at 390 nm (Narasimhulu, 1996). In this study, we observed that the binding of benzphetamine to P450 2B6 was significantly affected as a result of the inactivation by PCP. The modification by PCP resulted in the affinity of benzphetamine for the enzyme being decreased as indicated by an approximately 7-fold increase in the spectral binding constant, Ks.
To determine whether the kinetics of binding were altered because of the inactivation, stopped-flow analysis was used, and the data revealed that the rate of benzphetamine binding was biphasic for the native protein with the second phase being the slower step. The PCP-inactivated 2B6 showed similar biphasic binding behavior; however, a larger fraction of the total change was seen in the second phase, and the rate in this phase was decreased by 15-fold compared with the native protein. When the overall rates for benzphetamine binding to the native and PCP-inactivated 2B6 were calculated, the data revealed striking differences in the rates of binding, and the results corresponded reasonably to the loss in the rates of 7-EFC O-deethylation activity (69 versus 60%). Thus, it is possible that the inactivation is primarily caused by a modification of the active site of 2B6, which slows down the rate of benzphetamine binding, possibly as a result of steric hindrance that leads to binding of benzphetamine in a different orientation than that which occurs with the native enzyme. Therefore, these active site modifications seem to be responsible for changes in substrate binding affinity, the kinetics of substrate binding, and possibly to changes in the orientation of the substrate in the active site.
Analysis of the profile for benzphetamine metabolism before and after the inactivation of 2B6 by PCP showed a significant decrease in two of the major metabolites, whereas the rates of formation of the other metabolites were not significantly affected. These data are consistent with the suggestion that benzphetamine binds in a different orientation in the active site as a result of the covalent modification by PCP, which may also be responsible for the decreased binding affinity of benzphetamine and slower binding kinetics. To determine the identity of other step(s) that might contribute to the decrease in the rate of metabolism after modification by PCP, we investigated other steps in the catalytic cycle.
It has previously been shown that mechanism-based inactivators of rat P450 2B1 lead to a decrease in the rate of reduction of the enzyme when investigated under anaerobic conditions (Roberts et al., 1995a; Yanev et al., 2000). Our results show that PCP-inactivated P450 2B6 is still able to form the reduced CO complex but that the overall rate of reduction is reduced by approximately 80% compared with the native enzyme. This is another striking change in the behavior of P450 2B6 as a result of active site modification by PCP. This decrease in the rate of reduction of the inactive enzyme reflects a slower rate of transfer of the first electron from NADPH via reductase to the P450, which could at least partially account for the observed decrease in the rate of enzymatic activity as seen for the PCP-inactivated 2B6.
We also determined the extent of uncoupling of NADPH oxidation from product formation for the native and PCP-inactivated enzymes. Several previous studies have used the ratios for the stoichiometry of NADPH oxidation to product and H2O2 formation as a measure of the extent of uncoupling of P450-catalyzed reactions (4, 6, 9, and 12). In the presence of the substrate EB, the native enzyme was approximately 67% efficient in using electrons from NADPH for the conversion of substrate to product, whereas 31% of the reducing equivalents were uncoupled from product formation and led to the production of H2O2. Under the same experimental conditions, the PCP-inactivated 2B6 exhibited a decrease overall in its ability to oxidize NADPH (73% relative to the native enzyme). In addition, only 30% of reducing equivalents were used in the conversion of substrate to product, whereas 70% of the reducing equivalents led to H2O2 formation. Thus, for EB the extent of uncoupling increased by more than a factor of 2, or, conversely, the efficiency of the coupling of electron transfer to product formation decreased by more than 2-fold. Because the percentage of reducing equivalents used for product formation may be substrate-dependent, these values may be different for benzphetamine. These data show that there is a significant increase in the uncoupling of the P450 catalytic cycle as a consequence of the covalent modification of 2B6 by PCP.
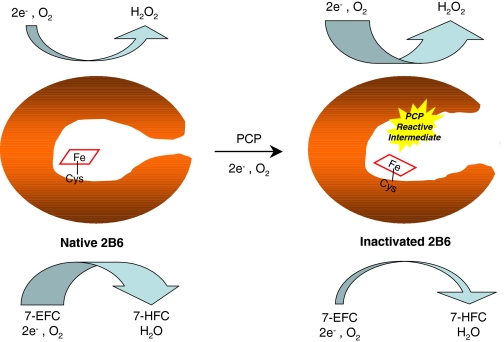
In conclusion, the covalent modification of the P450 2B6 by PCP occurs via a reactive intermediate that interacts with the apoprotein and results in the modification of the enzyme's catalytic function by compromising at least three steps in the P450 catalytic cycle. These modifications include impairment of the substrate binding step, a significant reduction in the rate of transfer of electrons from the reductase to the ferric enzyme, and an increase in the uncoupling of NADPH utilization from product formation. Figure 6 shows a cartoon proposed to explain the effects of inactivation of P450 2B6 by PCP where covalent modification of the apoprotein leads to changes in the overall structure of the active site and/or substrate access channels, and consequently the environment in the active site around the heme moiety is altered, resulting in the dramatic changes in the catalytic properties discussed above.
Fig. 6.
Cartoon showing the effects of mechanism-based inactivation of P450 2B6 by PCP. The covalent modification of the active site by a PCP-reactive intermediate results in changes in the structure of the enzyme by possibly opening the active site, which allows for uncoupling of the electrons from product formation leading to increased formation of hydrogen peroxide. 7-EFC, 7-ethoxy-4-trifluoromethylcoumarin; 7-HCF, 7-hydroxy-4-trifluoro-methyl-coumarin; Cys-S, the axial cysteine ligand.
Acknowledgments
We thank Hsia-Lien Lin for purification of the reductase.
This work was supported in part by the National Institutes of Health National Cancer Institute [Grant CA16954]; the National Institutes of Health National Institute of General Medical Sciences [Grant GM20877]; and Merck.
Article, publication date, and citation information can be found at http://dmd.aspetjournals.org.
doi:10.1124/dmd.108.024661.
ABBREVIATIONS: P450, cytochrome P450; PCP, phencyclidine; 7-EFC, 7-ethoxy-4-trifluoromethylcoumarin; EB, ethylbenzene; KPi, potassium phosphate; DMSO, dimethyl sulfoxide; Ks, spectral binding constant; PCA, protocatechuic acid.
References
- Backes WL and Eyer CS (1989) Cytochrome P-450 LM2 reduction. Substrate effects on the rate of reductase-LM2 association. J Biol Chem 264 6252–6259. [PubMed] [Google Scholar]
- Buters JT, Schiller CD, and Chou RC (1993) A highly sensitive tool for the assay of cytochrome P450 enzyme activity in rat, dog and man. Direct fluorescence monitoring of the deethylation of 7-ethoxy-4-trifluoromethylcoumarin. Biochem Pharmacol 46 1577–1584. [DOI] [PubMed] [Google Scholar]
- Code EL, Crespi CL, Penman BW, Gonzalez FJ, Chang TK, and Waxman DJ (1997) Human cytochrome P4502B6: interindividual hepatic expression, substrate specificity, and role in procarcinogen activation. Drug Metab Dispos 25 985–993. [PubMed] [Google Scholar]
- Estabrook RW and Werringloer J (1978) The measurement of difference spectra: application to the cytochromes of microsomes. Methods Enzymol 52 212–220. [DOI] [PubMed] [Google Scholar]
- Eyer CS and Backes WL (1992) Relationship between the rate of reductase-cytochrome P450 complex formation and the rate of first electron transfer. Arch Biochem Biophys 293 231–240. [DOI] [PubMed] [Google Scholar]
- Gorsky LD, Koop DR, and Coon MJ (1984) On the stoichiometry of the oxidase and monooxygenase reactions catalyzed by liver microsomal cytochrome P-450. Products of oxygen reduction. J Biol Chem 259 6812–6817. [PubMed] [Google Scholar]
- Gruenke LD, Konopka K, Cadieu M, and Waskell L (1995) The stoichiometry of the cytochrome P-450-catalyzed metabolism of methoxyflurane and benzphetamine in the presence and absence of cytochrome b5. J Biol Chem 270 24707–24718. [DOI] [PubMed] [Google Scholar]
- Halpert JR, Miller NE, and Gorsky LD (1985) On the mechanism of the inactivation of the major phenobarbital-inducible isozyme of rat liver cytochrome P-450 by chloramphenicol. J Biol Chem 260 8397–8403. [PubMed] [Google Scholar]
- Hanna IH, Reed JR, Guengerich FP, and Hollenberg PF (2000) Expression of human cytochrome P450 2B6 in Escherichia coli: characterization of catalytic activity and expression levels in human liver. Arch Biochem Biophys 376 206–216. [DOI] [PubMed] [Google Scholar]
- Hildebrandt AG, Roots I, Tjoe M, and Heinemeyer G (1978) Hydrogen peroxide in hepatic microsomes. Methods Enzymol 52 342–350. [DOI] [PubMed] [Google Scholar]
- Hollenberg PF, Kent UM, and Bumpus NN (2008) Mechanism-based inactivation of human cytochromes P450s: experimental characterization, reactive intermediates, and clinical implications. Chem Res Toxicol 21 189–205. [DOI] [PubMed] [Google Scholar]
- Jushchyshyn MI, Kent UM, and Hollenberg PF (2003) The mechanism-based inactivation of human cytochrome P450 2B6 by phencyclidine. Drug Metab Dispos 31 46–52. [DOI] [PubMed] [Google Scholar]
- Kent UM, Pascual L, Roof RA, Ballou DP, and Hollenberg PF (2004) Mechanistic studies with N-benzyl-1-aminobenzotriazole-inactivated CYP2B1: differential effects on the metabolism of 7-ethoxy-4-(trifluoromethyl)coumarin, testosterone, and benzphetamine. Arch Biochem Biophys 423 277–287. [DOI] [PubMed] [Google Scholar]
- Narasimhulu S (1996) Interactions of substrate and product with cytochrome P450 2B4. Biochemistry 35 1840–1847. [DOI] [PubMed] [Google Scholar]
- Reed JR and Hollenberg PF (2003) Comparison of substrate metabolism by cytochromes P450 2B1, 2B4, and 2B6: relationship of heme spin state, catalysis, and the effects of cytochrome b5. J Inorg Biochem 93 152–160. [DOI] [PubMed] [Google Scholar]
- Roberts ES, Ballou DP, Hopkins NE, Alworth WL, and Hollenberg PF (1995a) Mechanistic studies of 9-ethynylphenanthrene-inactivated cytochrome P450 2B1. Arch Biochem Biophys 323 303–312. [DOI] [PubMed] [Google Scholar]
- Roberts ES, Hopkins NE, Zaluzec EJ, Gage DA, Alworth WL, and Hollenberg PF (1995b) Mechanism-based inactivation of cytochrome P450 2B1 by 9-ethynylphenanthrene. Arch Biochem Biophys 323 295–302. [DOI] [PubMed] [Google Scholar]
- Sams C, Loizou GD, Cocker J, and Lennard MS (2004) Metabolism of ethylbenzene by human liver microsomes and recombinant human cytochrome P450s (CYP). Toxicol Lett 147 253–260. [DOI] [PubMed] [Google Scholar]
- Shebley M, Jushchyshyn MI, and Hollenberg PF (2006) Selective pathways for the metabolism of phencyclidine by cytochrome P450 2b enzymes: identification of electrophilic metabolites, glutathione, and N-acetyl cysteine adducts. Drug Metab Dispos 34 375–383. [DOI] [PubMed] [Google Scholar]
- Yanev SG, Kent UM, Roberts ES, Ballou DP, and Hollenberg PF (2000) Mechanistic studies of cytochrome P450 2B1 inactivation by xanthates. Arch Biochem Biophys 378 157–166. [DOI] [PubMed] [Google Scholar]