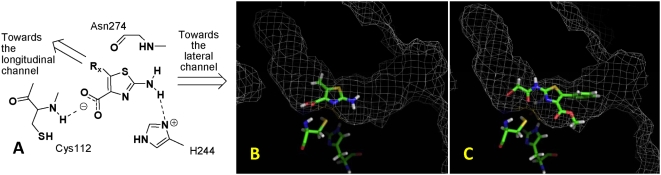
Figure 2. The modeling studies of the 2-aminothiazole-4-carboxylate analogues with mtFabH.
(A) The hypothetical template of the 2-aminothiazole-4-carboxylates for mtFabH inhibitor development. This illustrates the key H-bonding interactions with the catalytic triad amino acid residues. (B) The binding pose of methyl 2-amino-5-methylthiazole-4-carboxylate in the active site of mtFabH showing the NH2 group proximal to His 244 and directed towards the lateral channel, with the 5-methyl group directed towards the longitudinal channel. (C) The binding pose of methyl 2-(2-bromoacetamido)-5-(3-chlorophenyl)thiazole-4-carboxylate with the bromomethylene portion in the vicinity of the Cys112 thiol group.