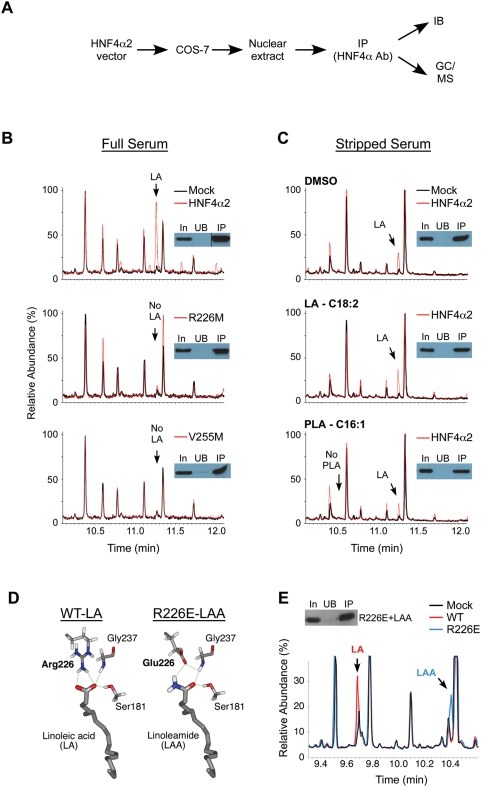
Figure 1. Identification of linoleic acid (LA) as the endogenous ligand for mammalian-expressed HNF4α2.
(A) Affinity isolation/mass-spectrometry (AIMS): rat HNF4α2 was purified by immunoprecipitation (IP) from crude nuclear extracts of transfected COS-7 cells using an HNF4α specific antibody (HNF4α Ab). The amount of HNF4α2 protein recovered was determined by immunoblot (IB) analysis and bound ligands bound were identified by GC/MS. (B) GC/MS chromatograms (10 to 12 min) comparing compounds extracted from IP'd material from mock-transfected (black) to HNF4α2-transfected (red) (wt and LBP mutants R226M and V255M) COS-7 cells grown in 10% bovine calf serum (Full Serum). Insets: HNF4α IB showing input (In, 2% of total), material not bound by the Protein A Sepharose (unbound, UB, 2%), and IP'd material (IP, 4%). (C) Same as in (B) except lipid-depleted serum (Stripped Serum) was used and vehicle (DMSO), linoleic acid (LA, 30 µM) or palmitoleic acid (PLA, 30 µM) were added to the media as indicated. Arrow, position of LA and PLA peak as determined by standards. (D) Predicted model of HNF4α2 (WT) with bound LA showing the hydrogen bonding between the Arg226 guanidinium group and the LA carboxylate head group (left panel). Right panel, model of HNF4α R226E mutant bound to linoleamide (LAA) with the carboxylate group of the Glu226 mutant interacting with the LAA NH2 head group. (E) GC/MS chromatogram comparison and corresponding IB of HNF4α2 WT (red) and R226E mutant (blue) IP'd from COS-7 cells treated with 30 µM LAA. LA and LAA peaks are indicated.