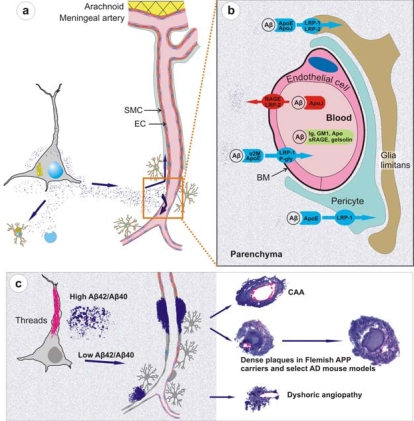
Figure 3.
Physiological clearance and pathological deposition of Aβ in brain.
(a) The newly synthesized Aβ is locally degraded by glial uptake and cell-associated and extracellular proteases or cleared along the periarterial interstitial fluid pathway or directly across the BBB through specific receptors or carrier-mediated mechanisms (EC, endothelial cell; SMC, smooth muscle cell). (b) At the level of BBB, Aβ is transported into the blood flow via a transcytosis mechanism mediated by LRP-1 and P glycoprotein transporters, in association with α2 macroglobulin and ApoE. A reverse transport from the blood towards the parenchyma is mediated by RAGE and LRP-2 receptors in association with apolipoprotein J. In addition, Aβ might be sequestered in the blood flow by immunoglobulins, ganglioside GM1, apolipoproteins, soluble RAGE receptors and gelsolin. (c) Astrocyte endfeet and pericytes also mediate Aβ intake by expressing LRP-1 and -2 receptors. As the production of Aβ exceeds its clearance, Aβ starts to deposit. It is possible that in situations of high Aβ42/Aβ40 ratio, highly fibrillogenic Aβ42 deposits near the site of production as diffuse plaques. It is also likely that such plaques sequester newly synthesized Aβ and mature to dense plaques. However, in situations where Aβ42/Aβ40 ratio remains unaltered or low, the major Aβ gradient is set towards vessels where Aβ42 seeds the deposition of more abundantly produced, and more diffusible, Aβ40 to form vessel-related CAA and dense plaques. Figure is not drawn to scale (adapted from Pirici et al., with permission from the publisher [118].