Abstract
Although it is possible that binge eating in humans is due to increased responsiveness of orosensory excitatory controls of eating, there is no direct evidence for this because food ingested during a test meal stimulates both orosensory excitatory and postingestive inhibitory controls. To overcome this problem, we adapted the modified sham feeding technique (MSF) to measure the orosensory excitatory control of intake of a series of sweetened solutions. Previously published data showed the feasibility of a “sip-and-spit” procedure in nine healthy control women using solutions flavored with cherry Kool Aid® and sweetened with sucrose (0-20%)1. The current study extended this technique to measure the intake of artificially sweetened solutions in women with bulimia nervosa (BN) and in women with no history of eating disorders. Ten healthy women and 11 women with BN were randomly presented with cherry Kool Aid® solutions sweetened with five concentrations of aspartame (0, 0.01, 0.03, 0.08 and 0.28%) in a closed opaque container fitted with a straw. They were instructed to sip as much as they wanted of the solution during 1-minute trials and to spit the fluid out into another opaque container. Across all subjects, presence of sweetener increased intake (p<0.001). Women with BN sipped 40.5-53.1% more of all solutions than controls (p=0.03 for total intake across all solutions). Self-report ratings of liking, wanting and sweetness of solutions did not differ between groups. These results support the feasibility of a MSF procedure using artificially sweetened solutions, and the hypothesis that the orosensory stimulation of MSF provokes larger intake in women with BN than controls.
Keywords: Modified sham feeding, bulimia nervosa, sip and spit, eating disorders, orosensory stimuli, hedonics, sweet solutions, aspartame, artificial sweetener
Background
Bulimia Nervosa (BN) is an eating disorder of unknown etiology that is characterized by consumption of abnormally large meals with subjective loss of control (“binge-eating episodes”). While research in BN suggests that abnormalities in inhibitory controls of eating are present (e.g., CCK abnormalities; abnormalities in gastric capacity and function2), it is also possible that binge eating in this disorder is due to increased responsiveness of orosensory excitatory controls of eating. Direct evidence for this hypothesis is difficult to obtain because food ingested during a test meal stimulates both orosensory excitatory and postingestive inhibitory controls3. To overcome this problem, we have adapted the modified sham feeding technique (MSF; e.g.4-8) to measure the orosensory excitatory control of intake of a series of sweetened solutions.
We recently described the use of MSF to measure the intake of five solutions, unsweetened and sweetened with four concentrations of sucrose, in nine healthy women without eating disorders1. By limiting the sweet stimuli to the mouth, the MSF technique measures the orosensory stimulation of intake by sweet taste in the absence of postingestive inhibitory stimulation. Intake of each solution, measured for two minutes of MSF, was followed immediately by self reports of the perceived intensities of sweetness and liking of that solution using visual analogue scales. This experimental design provided a novel method for investigating the orosensory effect of sweetener concentration on intake and for investigating the associations among sweetness, liking and intake of solutions with five concentrations of sucrose (0-20%) under these conditions.
The current study uses MSF to test the hypothesis that women with BN will ingest more solution during the orosensory stimulation of MSF than women without histories of an eating disorder.
Methods
Eleven healthy women with no history of eating disorder or other psychiatric illness and 13 women meeting criteria for BN (as defined in DSM-IV9) were recruited for study participation from the surrounding community, university, and medical center by flyers and newspaper and internet advertisements. All women were between ages 18-40 years, with current body weight ranging from 80%-120% ideal body weight by Metropolitan Life Insurance Tables10. Table 1 shows baseline data including subjects' age, body mass index (BMI), illness duration (for participants with BN), weight suppression (difference between lifetime maximum and lifetime minimum weights), total scores on Eating Disorder Examination (EDE11) and Beck Depression Inventory (BDI12), and self-reported average weekly use of selected artificially sweetened products including chewing gum, “diet” beverages, and packets of artificial sweetener. The Eating Disorder Examination is a semi-structured diagnostic interview that quantifies psychological and behavioral symptoms of eating disorders and the Beck Depression Inventory is a 21-question self-report survey of depressive symptomatology.
Table 1. Clinical and Demographic Characteristics of Subjects.
| Subject Group | Age (years) | BMI (kg/m2) | Illness Duration (years) | Weight Suppression (pounds) | EDE Total score | BDI Total score | Weekly servings gum (pieces) | Weekly 12-oz servings diet drink | Weekly Artificial Sweetener Packets |
|---|---|---|---|---|---|---|---|---|---|
| Bulimia Nervosa (BN) | 24.45 (1.55) | 22.46 (0.83) | 9.7 (2.37) | 45.25 (7.8)** | 4.07*** (0.26) | 18.50*** (1.90) | 33.05 (15.29) | 18.67* (5.63) | 29.00 (13.62) |
| Normal Control (NC) | 26.7 (1.4) | 21.01 (0.66) | N/A | 16.7 (1.7) | 0.16 (0.05) | 1.40 (0.54) | 3.41 (1.56) | 2.63 (1.25) | 3.13 (2.58) |
Data for age and BMI are mean ±(SE) from 11 BN and 10 NC subjects. Data for illness duration, weight suppression, EDI and BDI are from 10 BN and 10 NC subjects; data for weekly servings of artificially sweetened products are from 10 BN and 9 NC subjects.
Weight Suppression = Difference between lifetime maximum and lifetime minimum weight
EDE = Eating Disorder Examination
BDI = Beck Depression Inventory
Weekly servings of diet products was based on subject recall of prior 4 weeks' use.
significantly more drinks, p<0.05;
significantly larger difference, p<0.005;
significantly higher score, p<0.005.
Subjects were told they were participating in a study designed to test the response of people with and without eating disorders to the taste of food without swallowing it. The experimental procedure was conducted over a single one-hour period in the early afternoon, three to four hours after eating a standardized breakfast (English muffin, pat of butter, 6 oz apple juice; approximately 300 kcal). Subjects were instructed to sip the solution from the container on their left and to spit it into the container on their right, without holding it in their mouths, swishing it around, or swallowing it. Subjects were told that the rate at which they sipped and spit was entirely up to them and that there was no requirement or expectation for them to sip all of the solution presented.
Prior to the experimental session, subjects were asked to rate on visual analogue scales (VASs) their perceived sweetness, liking and wanting of each of the five experimental solutions after tasting and spitting out a small sample of each. These VASs consisted of pencil-and-paper assessments including the following questions: “How much did you LIKE what you just tasted?” “How much do you WANT MORE of what you just tasted?” and “How SWEET did what you just tasted seem to you?”. Beneath each question was a 10-cm horizontal line, anchored at either end by “Not at all” and “Extremely.” Subjects were asked to indicate their answers to these questions by placing a vertical mark along the horizontal line to estimate their experiences. VASs for each solution were on a separate piece of paper. Prior to beginning the experimental session, subjects completed an additional series of VASs to rate their perceived hunger, desire to eat, desire to binge, desire to vomit and anxiety. Table 2 depicts ratings of these baseline measures by BN and NC participants. Subjects were also given a one-minute training session during which they practiced the sipping and spitting technique using water.
Table 2. Baseline VAS Ratings of Subjects.
| Subject Group | Hunger (cm) | Desire to Eat | Desire to Binge | Desire to Vomit | Anxiety |
|---|---|---|---|---|---|
| BN | 5.61 (0.45) | 5.87 (0.49) | 2.25 (0.99) | 1.17 (0.85) | 4.56 (0.89)* |
| NC | 5.40 (0.62) | 5.79 (0.61) | 0.21 (0.05) | 0.19 (0.06) | 1.16 (0.55) |
Ratings were obtained immediately prior to sipping and spitting solutions. Data are mean ±(SE) of VAS ratings from 10 BN and 9 NC subjects.
significantly higher, p=0.006.
Following the training session, subjects were given access to each of 15 solutions that they were instructed to sip and spit for one minute. The solutions were presented in three sets, each containing five solutions of different aspartame concentrations (0, 0.145, 0.3, 0.75, and 2.8 g/L, or 0, 0.01, 0.03, 0.08 and 0.28% wt/wt, respectively) in distilled water, flavored with a constant concentration of cherry Kool Aid® (1.902 grams per Liter). Kool Aid® was added to make the solutions more palatable and more comparable to beverages commonly consumed in the U.S. Solutions were prepared fresh 18-24 hours prior to each experimental day. Kool Aid® was purchased in 13 oz (3.6g) packets from NetGrocer.com. Aspartame was obtained from Ajinomoto USA, Inc., Paramus, NJ. Aspartame, a low-calorie sweetener, was substituted for sucrose in the current paradigm because pilot testing using sucrose solutions with women with eating disorders suggested that their concerns about the caloric content of sucrose solutions limited their intake, despite instructions to not swallow the solutions. Aspartame concentrations were selected to provide approximate sweetness intensity of sucrose solutions of 0, 2.5, 5, 10 and 20%, where 10% sucrose approximates the sweetness of commercially available sugar-sweetened carbonated beverages13. All participants were specifically informed that the experimental solutions contained no sugar and no calories.
Within each set, the five flavored solutions (14.4-15.6 °C) were presented in a random order. Nineteen hundred mL of each solution were presented in an identical, opaque, unmarked, closed container that prevented visualization of the volume of the solution during the one-minute test (two L were prepared of each solution from which 100 mL were drawn off to provide samples for the taste test). Identical containers were used to collect the liquid spit out. Subjects sipped solutions through a straw and spit the oral contents out immediately into a funnel in the top of the spit container. Subjects were observed by experimenters using a Lorex™ four-channel closed-circuit observation system (Strategic Vista International, Inc., Baltimore, MD) and were signaled to start and stop sipping and spitting by a doorbell tone. Signaling was performed by an observer, who monitored the time using a digital timer.
There was a one-minute interval between presentations of solutions. Immediately after each one-minute trial, subjects used VASs to report their perceived intensities of sweetness, wanting, and liking of the solution, as well as their anxiety and desire to eat, binge and vomit. Then they rinsed their mouths with a solution consisting of baking soda dissolved in distilled water (23.7g per 1000ml distilled H2O). For VASs, separate sheets of paper were used for each time point and subjects did not have access to their previous responses. Responses were measured to the nearest millimeter using a centimeter ruler.
The sip and spit containers were weighed before and after each one-minute trial measured to the nearest 0.1 gram using an Acculab L-Series 7200 scale (Acculab, Edgewater, NY). The grams sipped or spit was the difference in the weight of the containers before and after each trial.
After the entire test was completed, subjects were debriefed and asked about their expectations of the experimental hypotheses, ability to comply with the experimental instructions, and experience of the procedure.
One patient with BN reported misunderstanding study instructions and thinking that the solutions were prepared with sucrose and not artificial sweetener. This patient sipped minimal quantities of solution and attributed this to fear of caloric absorption; her data were excluded. Another patient with BN reported the solutions to taste “bitter” and on further questioning endorsed extreme sensitivity to bitter tastes in, and avoidance of, artificial sweetener as well as various vegetables and other foods; this pattern is thought to be consistent with “supertaster” status14 and her data were also excluded from analyses. No other participants described solutions as tasting bitter. One control participant did not comply with instructions to rinse between solutions despite being repeatedly asked to do so and her data were also excluded. Thus, the data from 11 BN and 10 NC subjects were analyzed.
All of the experimental procedures were approved by the Institutional Review Board of the New York State Psychiatric Institute and conducted according to NIH guidelines of Good Clinical Practice.
Statistical Analysis
Repeated measures ANOVA was used to analyze intake and VAS measures of the perceived intensities of sweetness, liking and wanting as a function of trial (1, 2 or 3) and aspartame concentration, using diagnostic group (BN vs NC) as the between-group variable. Significant treatment effects were analyzed post hoc by the Least Significant Difference (LSD) Test. Differences were considered significant when p<0.05. Greenhouse-Geisser correction was performed on all ANOVA results to correct for dependence among observations within subjects. T-tests were used to compare intake and VAS ratings between BN and NC subjects at each trial of each concentration.
Separate Pearson product-moment correlation coefficients were determined for the relationships among intake and perceived sweetness, liking and wanting across aspartame concentrations and across trials, within each group of subjects (BN subjects and NC subjects), and among individual subjects. Separate Pearson correlation coefficients were also determined for the relationships between intake and VAS ratings of hunger, desire to eat, desire to binge, desire to vomit, and anxiety obtained at baseline (prior to sipping and spitting first flavored solution), as well as clinical measures including subject age, BMI, lifetime maximum and minimum BMI, self-reported use of artificially sweetened products in the preceding month, and, for BN participants, duration of the eating disorder. Because of significant group differences on several of these measures, correlation among clinical and behavioral measures were determined within each diagnostic group separately. VAS data were not available for one patient with BN and one control. Full clinical data were not available for one patient with BN and information about use of artificially sweetened products was unavailable for one participant with BN and one control.
Results
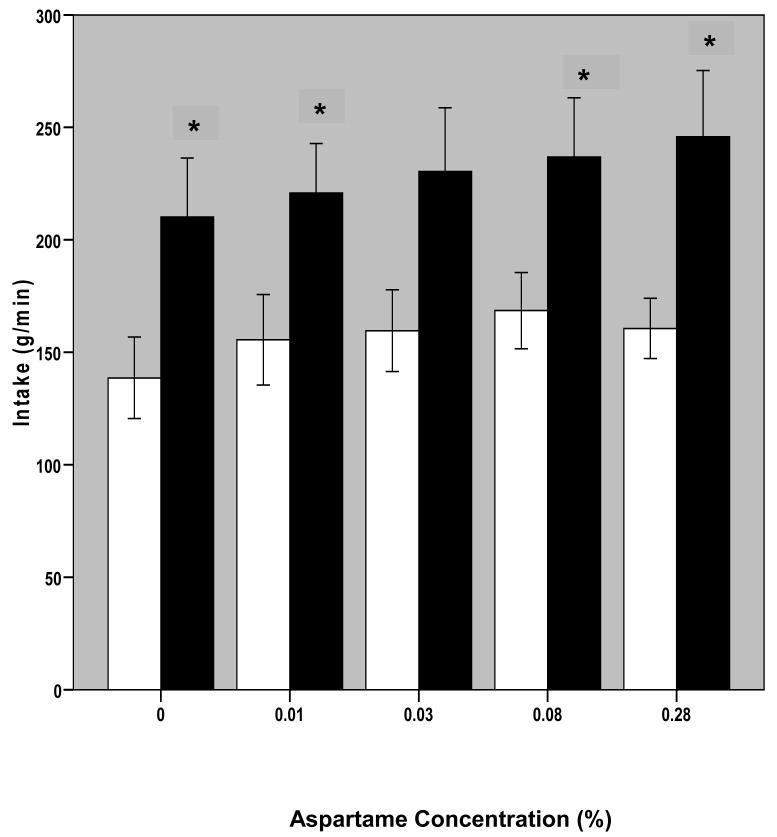
The major result of the study is that BN participants ingested significantly more of the solutions than NC subjects during MSF (F [1,19] = 5.23, p =0.034; Figure 1). Intake in MSF was also a function of sweetener concentration (F[2.98, 56.69]=7.38, p<0.001). Post-hoc analysis revealed that the significant effect of concentration was attributable to differences between the unsweetened solution and all other solutions and between solutions 2 and 4 (p values <0.05). Intake averaged across the three trials for BN subjects was significantly larger than the intake of NC at each solution (aspartame concentration; p < 0.05) except for the 0.03% aspartame solution, in which the larger intake of BN subjects was not quite significant (p = 0.054).
Figure 1. Intake by Sweetness Intensity (Aspartame Concentration).
Bars represent mean +/- 1 S.E. (error bars) grams of solution sipped, averaged over the three one-minute trials at each aspartame concentration. Blank bars are for NC and black bars are for BN subjects.
*significantly greater intake by BN compared with NC subjects, p<0.05
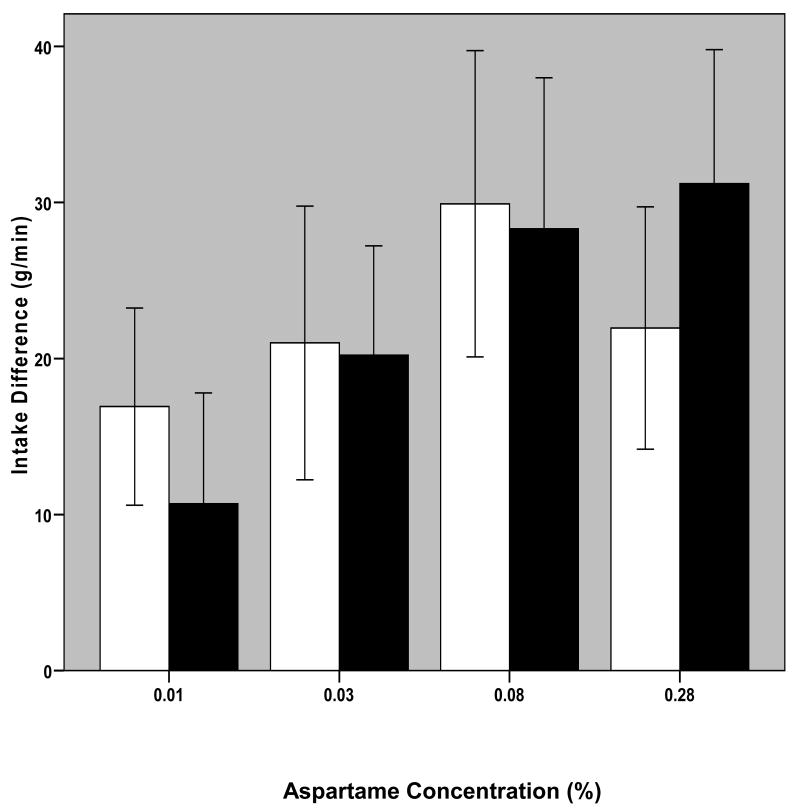
Sweetness was not necessary for the larger intake in BN because they also ingested significantly more of the unsweetened solution (0%) than NC (p < 0.05; Figure 1). To determine if the difference in intake of this solution accounted for any of the differences in response to aspartame, two transformations were performed on intake data: first, intake of each sweetened solution as a function of difference from baseline (0% aspartame solution) intake was determined, and second, percent difference in intake of each of the sweetened solutions compared with baseline intake was calculated. One-way ANOVA with repeated measures (four solution concentrations and three trials) was performed on these data. No significant effect of trial or of concentration were found on data transformed in either way, and t-tests on transformed data also revealed no differences between BN and NC subjects (Figure 2). Furthermore, no difference was found between BN and NC groups for slopes (B coefficient in linear regression) calculated for the effect of aspartame concentration on intake or on intake difference.
Fig. 2. Difference from Intake of 0% Aspartame Solution.
Bars represent mean (+/- 1 S.E.) difference in intake of each of the sweetened solutions (0.01-0.28%) and the 0% aspartame solution, in grams, averaged over the three one-minute trials. Blank bars are for NC and black bars are for BN subjects.
The concentration of aspartame that elicited the largest intake varied both within and between groups. Among BN subjects, the modal solution of maximal intake was the sweetest solution: two showed maximal intake of the 0.01% aspartame solution, one showed maximal intake of the 0.03% aspartame solution, three had maximal intake of the 0.08% aspartame solution and five of the 0.28% aspartame solution. Among NC subjects, the modal solution of maximal intake was the second-sweetest solution (0.08% aspartame): two showed maximal intake of the 0.01% aspartame solution, one of the 0.03% aspartame solution, five of the 0.08% aspartame solution, and two of the 0.28% aspartame solution. No subject had maximal intake of the 0% aspartame solution. As per above, paired t-tests within each group showed no significant differences between intake of the two sweetest solutions (for BN participants: t[df=10]=-1.116, p=0.291; for NC participants, t[df=9]=1.013, p=0.338). Thus, there was no significant difference between the ingestive responses of BN and NC to increasing concentrations of aspartame. The difference between groups appears to be due to the significantly larger baseline intake (i.e., intake of unsweetened solution) by participants with BN.
To investigate the possibility that NC subjects ingested less because they swallowed more and the larger amount swallowed decreased intake through postingestive negative feedback effects, we measured the difference between amount sipped and amount spit. No significant difference was found using paired t-tests among either group of subjects, and the amount swallowed (indicated by a positive value for sipped – spit) among control subjects was always less than 5% of amount sipped and was so small as to be unlikely to produce significant postingestive feedback (maximum among all NC subjects was 3.6% of total intake). BN subjects were more likely to swallow solutions than control subjects (i.e., average value for sipped – spit was positive) though the total amount swallowed over all trials did not exceed 7.8% of total solution intake and so similarly was so small as to be unlikely to produce significant postingestive feedback. The total amount in grams swallowed by BN and NC was not significantly different (t[df=13.1]=1.81, p=0.094; Table 3), nor was the total amount swallowed expressed as percentage of total intake (t[df=19]=1.94, p=0.067), though when this was examined at each solution, percentage of solutions swallowed by BN participants did slightly exceed percentage swallowed by controls at the 0.03 and 0.08% solutions (Table 3).
Table 3. Difference Between Amount of Solution Sipped and Solution Spit Across Trials.
| Subject Group | 0% Aspartame | 0.01% Aspartame | 0.03% Aspartame | 0.08% Aspartame | 0.28% Aspartame | |
|---|---|---|---|---|---|---|
| BN | g | 0.03 (0.97) | 2.28 (2.11) | 3.36 (2.44) | 6.11 (3.48) | 5.93 (3.57) |
| % | -0.19% (0.46) | 0.68% (0.90) | 1.37% (1.05)* | 2.41% (1.40)* | 2.30% (1.46) | |
| NC | g | -0.61 (0.84) | -0.08 (0.98) | -1.60 (1.27) | -1.25 (1.02) | -1.39 (0.92) |
| % | -0.89% (0.62) | -0.56% (0.66) | -1.35% (0.72) | -0.97% (0.58) | -1.06% (0.60) | |
Data are sum of differences between solution sipped and spit averaged across each of the three trials from each of the five solutions, in mean ±(SE) g (top value) and percent of solution sipped ±(SE) (bottom value). Positive value suggests swallowed solution. Negative value indicates that fluid spit exceeded solution sipped, consistent with salivation.
significantly larger difference in BN vs NC, p≤0.05.
VAS measures
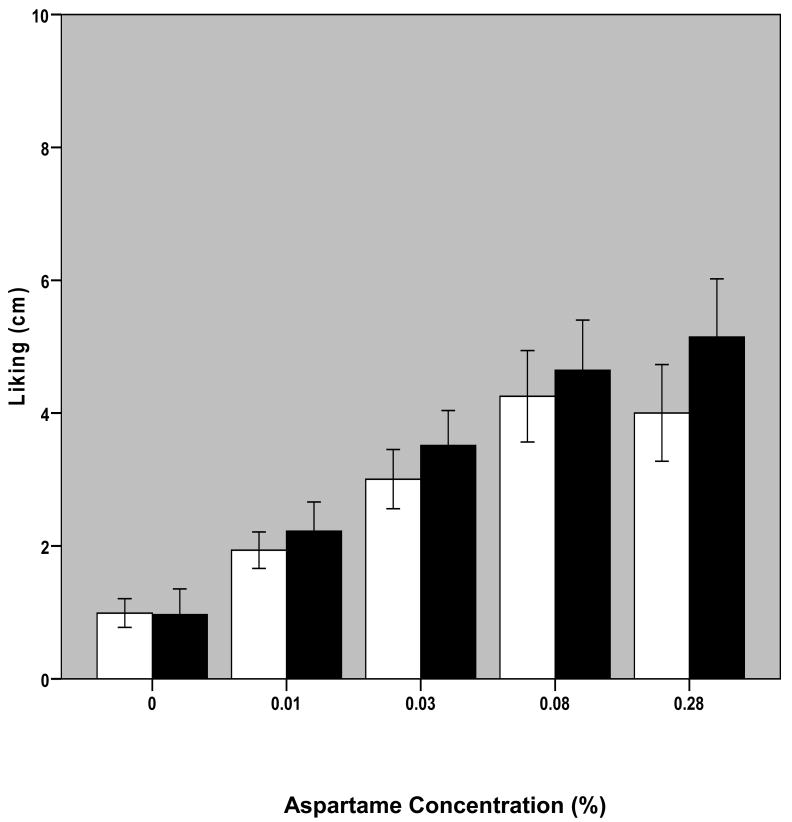
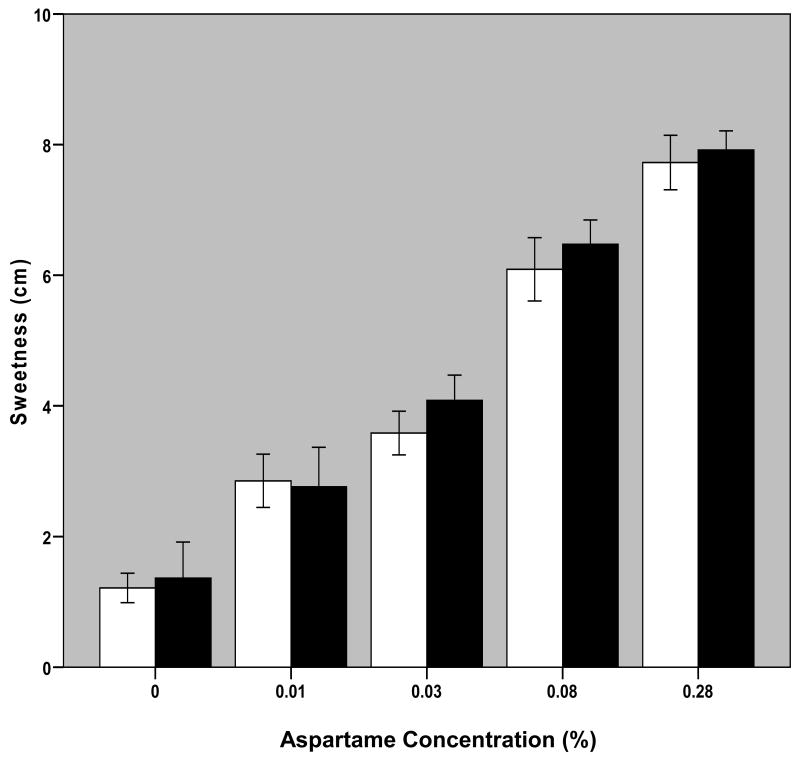
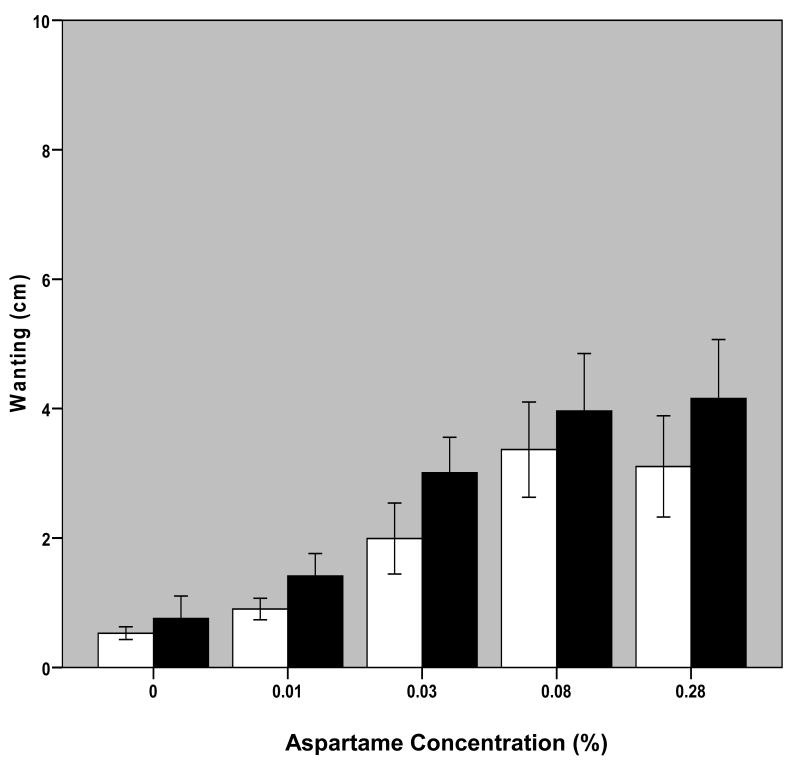
Changes in aspartame concentration significantly affected intake VAS reports of liking, wanting and perceived sweetness of solutions per repeated measures ANOVA (liking: F[1.81,34.34]=26.31, p<0.001; wanting: F[1.68,31.83]=19.60, p<0.001; sweetness: F[2.46,46.64]=112.4, p<0.001; Figures 3-5). There were no significant effects of diagnostic group or interactive effects between aspartame concentration and diagnostic group on these measures. Post-hoc analyses revealed significant differences (p<0.05) among all solutions in both perceived liking and wanting with the exception of solutions 4 versus 5. Differences were found among sweetness ratings of all solutions that were significant to p<0.010.
Fig. 3. Self-Reported Liking as a Function of Aspartame Concentration in Solution.
Bars represent mean +/- S.E. of self-reported liking ratings of solution at each aspartame concentration averaged over the three trials for 11 BN and 10 NC participants. Blank bars are for NC and black bars are for BN subjects.
Fig. 5. Perceived Sweetness as a Function of Aspartame Concentration in Solution.
Bars represent mean +/- S.E. of self-reported sweetness ratings of solution at each aspartame concentration averaged over the three trials for 11 BN and 10 NC participants. Blank bars are for NC and black bars are for BN subjects.
The modal solution that elicited maximal liking ratings among patients with BN was the 0.28% aspartame solution: two subjects gave highest liking ratings to the 0.03% aspartame solution; two subjects to the 0.08% aspartame solution, and six subjects to the 0.28% aspartame solution; the remaining subject provided identical liking ratings to solutions 5 and 2. The modal solution of maximal liking ratings among controls was the 0.08% aspartame solution: one participant provided maximal liking ratings to the 0.01% aspartame solution; two to the 0.03% aspartame solution; four to the 0.08% aspartame solution, and three to the 0.28% aspartame solution. The solution that elicited maximal wanting ratings among patients with BN was the 0.01% aspartame solution for one subject, the 0.03% aspartame solution for one subject, the 0.08% aspartame solution for 3 subjects and the 0.28% aspartame solution for six; among controls, one provided maximal wanting ratings for the 0% aspartame solution, two for the 0.01% aspartame solution, four for the 0.08% aspartame solution, and 3 for the 0.28% aspartame solution. The 0.28% aspartame solution elicited maximal sweetness ratings in all participants with the exception of three patients with BN who provided maximal sweetness ratings for the 0.08% aspartame solution.
To investigate the possibility that the difference in amount sipped between BN and NC subjects was due to differences in perceived sweetness, liking or wanting of the solutions, self-report assessments of these measures were considered. No significant differences were found on any of the VAS measures between BN and NC subjects, with the exception of “liking” and “wanting” of the 0.03% aspartame solution at trial 1.
Correlates of solution intake
Correlations among intake, liking, wanting, perceived sweetness
When intake, sweetness, liking and wanting data were averaged across all subjects within each diagnostic group for each of the five aspartame concentrations, significant correlations were found among intake and liking, wanting and sweetness among BN subjects (r=0.991, p=0.001; r=0.973, p=0.005; r=0.983, p=0.003, respectively). These associations were less robust among NC subjects (r=0.919, p=0.028; r=0.857, p=0.063; r=0.779, p=0.121, respectively). Within both subject groups, ratings of solution liking, wanting and sweetness were all highly correlated (r values 0.934-0.991, p<0.05 for all comparisons).
Considerably greater variation was found when these associations were examined within individual subjects. For example, the correlation between intake and liking ranged from r=-0.612 to 0.804, with a mean r=0.439 (SEM=0.159) in BN subjects, and ranged from r=-.508 to 0.997, with a mean r=0.449 (±0.166) in controls (NSD). The mean correlations between intake and wanting were 0.341 (±0.207) in BN subjects and 0.379 (±0.174) in controls, between intake and sweetness were 0.452 (±0.189) in BN subjects and 0.544 (±0.114) in controls, between sweetness and liking were 0.747 (±0.121) in BN subjects and 0.667 (±0.134) in controls, and between wanting and sweetness, 0.690 (±0.139) in BN subjects and 0.548 (±0.169) in controls (all NSD between groups). The association between liking and wanting, on the other hand, remained significant in all participants except three patients with BN and three controls, with mean r values of 0.901 (±0.053) among BN patients and 0.729 (±0.156) in controls (NSD between groups).
Baseline clinical measures and VAS measures, and their relationship to intake
Baseline VAS measures of anxiety were higher among patients with BN than controls and there was a trend towards higher baseline ratings of desire to binge among BN subjects compared with controls (Table 2). No differences were present in baseline self-reported hunger, desire to eat, or desire to vomit. Baseline clinical measures distinguished BN subjects from controls as follows (Table 1): weight suppression, as defined as the difference between lifetime (non-pregnant) maximum and minimum weights; total EDE score; BDI score, and self-reported weekly servings of diet beverages.
When intake measures (total intake across all trials and solutions) were compared with clinical and baseline VAS measures among control subjects, no association was found between intake and BMI, age, weight suppression, EDE or BDI score, weekly servings of gum, diet beverages or sweetener packets, or baseline VAS measures including hunger, desire to eat, desire to binge, desire to vomit, or anxiety. No associations were found between intake and baseline VAS measure among BN patients. Age was the only clinical factor found to significantly predict intake (r=0.810, p=0.005) among BN participants. Graphical inspection of this relationship revealed that one participant's data drive this association; when her data are excluded this relationship becomes non-significant.
Discussion
Previously we reported that a “sip-and-spit” MSF technique using solutions sweetened with sucrose was feasible and well tolerated in women without an eating disorder. The current study extends this technique to women with BN using aspartame solutions, demonstrating that women with BN exhibit approximately 50% greater intake of solutions in this novel eating-related behavioral paradigm than healthy control women. Several aspects of this study warrant further discussion.
The finding of greater intake by women with BN compared with controls is consistent with our hypothesis that women who binge eat are more responsive to orosensory stimuli. Our data failed to support the hypothesis that this responsiveness is specific to sweet stimuli: when corrected for baseline differences (i.e., intake of unsweetened solution) incremental responsiveness to increasing concentration of sweetener did not exceed that of controls. Thus, women with BN appear to exhibit a non-specific proclivity for this sip-and-spit feeding-related behavior. This is the first report of this finding. Factors that mediate this predilection are unclear, and warrant further investigation.
Mechanisms of increased intake in this MSF paradigm by BN participants presumably include the same cognitive, affective and learned factors that promote binge-eating and purging behavior in this population. Other “oral” behaviors such as cigarette smoking and use of chewing gum are reported to occur with increased frequency in people with eating disorders as well15;16, as is chewing and spitting out food17, which arguably represents a “naturally existing” form of sham feeding. That some artificially sweetened products similarly provide orosensory stimulation without postingestive volume and/or nutrient effects, and are used to a greater extent by individuals with eating disorders than those without16, including in this study, may be further evidence of increased responsiveness to orosensory sweet stimuli in individuals who binge eat and purge. It also raises the possibility that the habitual use of artificially sweetened foods (and/or the habitual vomiting after consumption of binge meals), in disrupting the normal conditioned association between sweet taste and caloric content, itself promotes dysregulation of appetite and/or eating behavior18 19. Women with BN may have an improved capability for sipping and spitting behavior as a function of differences in oral musculature and/or its innervation, as could be related to a “training” effect of experience with repetitive consumption of large quantities of food. It is also possible that some feature of MSF independent of orosensory stimulation elicits more behavior in women with BN, such as the repetitive aspect of sipping and spitting. It may be that higher levels of anxiety and/or depression, as were found in our BN participants, mediated their enhanced intake relative to controls, and would contribute to greater intake among non-eating disordered groups with anxiety and/or depression, as well. It is furthermore possible that abnormalities in salivary composition or flow or oral sensations in BN participants could contribute to greater intake of solution in this paradigm and of beverages in general20;21. Some other unmeasured factor unrelated to the diagnosis of BN may also have contributed to our main finding. These possibilities remain open to investigation.
Some, but not all, previous studies have demonstrated higher hedonic ratings for sweeter solutions in patients with BN compared with controls22,23, as would suggest that women with BN are more responsive specifically to sweet orosensory stimulation. These findings have not been universal, however, and discrepancies among studies may be due to differing degrees of deprivation prior to hedonic assessments and range of sweetness intensity of solutions sampled, among other factors22. The lack of significant difference in our study between subject groups on self-reported liking and wanting of solutions may be a function of these factors, and/or of our small sample size, and must be interpreted cautiously also because it is possible that the scale of BN is different than the scale of controls due to different experiences in the food world24. Use of labeled magnitude scaling in place of visual analogue scales would improve the validity of these measures25, as would routine assessment of taster status14. Extending the range of sweetness concentrations assessed might also demonstrate significant between-group differences. This might also enable the detection of differential behavioral responsiveness to increasing sweetness concentration between subject groups, as might consideration of individual differences or “sub-types” of sweetness responsivity within a larger study sample.
The lack of difference between self-reported liking and wanting of solutions within each group is notable as well, as the distinction between the hedonic and motivational effects of rewarding stimuli is an area of active investigation and some debate. It is argued by some that liking and wanting are mediated by different brain areas and neurochemicals and are distinguishable experimentally26,27. To the extent that liking and wanting are captured by the corresponding questions on the VASs our subjects completed, we did not measure a difference in these effects. Possible reasons for this include the temporal proximity of the questions, incomplete understanding on the part of subjects of the concepts of liking and wanting, and/or difficulty providing accurate self-assessment of these subjective states. It is also possible that actual beverage intake provided a more accurate measure of solution wanting than self-report measures. This could at least in theory be further examined experimentally through the use of pharmacological probes in this paradigm that have previously been suggested to differentially impact liking and wanting (e.g., opioidergic versus dopaminergic agents26,27), or use of additional methods to assess the reinforcing effect of sipping solutions in patients and controls. It may also be important to assess disliking of solutions in future studies.
In addition to the limitations described above, the current study is limited by a small sample size. Furthermore we have yet to demonstrate that spitting solutions is necessary to observe the large intakes exhibited in this paradigm (i.e., we did not compare solution intake in MSF to the quantity of solution subjects would drink under similar circumstances). However it offers the advantages of being an objective assessment of an eating-related behavior that minimizes the concerns of caloric ingestion and can be conducted in its entirety within a one-hour period. Future research could examine hormonal correlates and possible mediators of MSF behavior and could employ pharmacological and other experimental manipulations paralleling those that have been employed in MSF in rodents28-29;30, to explore mechanisms underlying excitatory controls of eating-related behavior in women with BN.
In conclusion, we present data to support the use of a novel “sip-and-spit” MSF paradigm assessing behavioral responses to solutions of varying sweetness intensity, to probe responsiveness to orosensory stimuli in a laboratory setting in women with and without eating disorders. The main finding of greater intake among women with BN than controls supports the validity and utility of this paradigm to detect heightened responsiveness to orosensory stimuli as appears to be characteristic of this behavioral disorder, the mediators and mechanisms of which are open to exploration.
Fig. 4. Self-Reported Wanting as a Function of Aspartame Concentration in Solution.
Bars represent mean +/- S.E. of self-reported wanting ratings of solution at each aspartame concentration averaged over the three trials for 11 BN and 10 NC participants. Blank bars are for NC and black bars are for BN subjects.
Acknowledgments
This work was supported by grants from the NIMH (71285, PI: Klein; 65024 and 79397, PI: Walsh). We would like to thank co-Investigators on the Translational Grant, colleagues in the EDRU and GCRU, and study participants for their contributions to this research. This work was presented in part at the 14th Annual Meeting of the Society for the Study of Ingestive Behavior in Naples, Florida, July 2006, at the 16th Annual Meeting of the Society for the Study of Ingestive Behavior in Paris, France, July 2008, and at the Columbia University Appetitive Behavior Seminar, March, 2008.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Klein DA, Schebendach JS, Devlin MJ, Smith GP, Walsh BT. Intake, sweetness and liking during modified sham feeding of sucrose solutions. Physiol Behav. 2006;87:602–606. doi: 10.1016/j.physbeh.2005.12.009. [DOI] [PubMed] [Google Scholar]
- 2.Hadley SJ, Walsh BT. Gastrointestinal disturbances in anorexia nervosa and bulimia nervosa. Curr Drug Targets CNS Neurol Disord. 2003;2:1–9. doi: 10.2174/1568007033338715. [DOI] [PubMed] [Google Scholar]
- 3.Smith GP. The direct and indirect controls of meal size. Neurosci Biobehav Rev. 1996;20:41–46. doi: 10.1016/0149-7634(95)00038-g. [DOI] [PubMed] [Google Scholar]
- 4.Teff K. Nutritional implications of the cephalic-phase reflexes: endocrine responses. Appetite. 2000;34:206–213. doi: 10.1006/appe.1999.0282. [DOI] [PubMed] [Google Scholar]
- 5.Richardson CT, Walsh JH, Cooper KA, Feldman M, Fordtran JS. Studies on the role of cephalic-vagal stimulation in the acid secretory response to eating in normal human subjects. J Clin Invest. 1977;60:435–441. doi: 10.1172/JCI108793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Feldman M, Richardson CT. Role of thought, sight, smell, and taste of food in the cephalic phase of gastric acid secretion in humans. Gastroenterology. 1986;90:428–433. doi: 10.1016/0016-5085(86)90943-1. [DOI] [PubMed] [Google Scholar]
- 7.Helman CA. Chewing gum is as effective as food in stimulating cephalic phase gastric secretion. Gastroenterology. 1986;90:428–433. [PubMed] [Google Scholar]
- 8.Smeets AJ, Westerterp-Plantenga MS. Oral exposure and sensory-specific satiety. Physiol Behav. 2006;89:281–286. doi: 10.1016/j.physbeh.2006.06.011. [DOI] [PubMed] [Google Scholar]
- 9.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th. 1994. [Google Scholar]
- 10.Metropolitan Life Insurance. New weight standards for men and women. Statistical Bulletin. 1959;40:1–4. [Google Scholar]
- 11.Fairburn CG, Cooper PJ. The Eating Disorder Examination in Binge Eating: Nature, Assessment, and Treatment. New York: Guilford Press; 1993. [Google Scholar]
- 12.Steer RA, Beck AT. Beck Depression Inventory (BDI) in Manual for the Beck Depression Inventory. San Antonio: Psychological Corporation; 1993. [Google Scholar]
- 13.Nutrient Data Laboratory, Beltsville Human Nutrition Research Center. USDA Database for the Added Sugars Content of Selected Foods. Release 1. Beltsville, MD: U.S. Department of Agriculture; 2006. [Google Scholar]
- 14.Lucchina LA, Curtis OF, 5th, Putnam P, Drewnowski A, Prutkin JM, Bartoshuk LM. Psychophysical measurement of 6-n-propylthiouracil (PROP) taste perception. Ann N Y Acad Sci. 1998:816–819. doi: 10.1111/j.1749-6632.1998.tb10666.x. [DOI] [PubMed] [Google Scholar]
- 15.Anzengruber D, Klump KL, Thornton L, Brandt H, Fichter MM, Halmi KA, Johnson C, Kaplan AS, LaVia M, Mitchell J, Strober M, Woodside DB, Rotondo A, Berrettini WH, Kaye WH, Bulik CM. Smoking in eating disorders. Eating Behaviors. 2006;7:291–299. doi: 10.1016/j.eatbeh.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 16.Klein DA, Boudreau GS, Devlin MJ, Walsh BT. Artificial sweetener use among individuals with eating disorders. Int J Eat Disord. 2006;39:341–345. doi: 10.1002/eat.20260. [DOI] [PubMed] [Google Scholar]
- 17.Guarda AS, Coughlin JW, Cummings M, Marinilli A, Haug N, Boucher M, Heinberg LJ. Chewing and spitting in eating disorders and its relationship to binge eating. Eat Behav. 2004;5:231–239. doi: 10.1016/j.eatbeh.2004.01.001. [DOI] [PubMed] [Google Scholar]
- 18.Swithers SE, Davidson TL. A Role for Sweet Taste: Calorie Predictive Relations in Energy Regulation by Rats. Behavioral Neuroscience. 2008;122:161–173. doi: 10.1037/0735-7044.122.1.161. [DOI] [PubMed] [Google Scholar]
- 19.Appleton KM, Blundell JE. Habitual high and low consumers of artificially-sweetened beverages: effects of sweet taste and energy on short-term appetite. Physiol Behav. 2007;92:479–486. doi: 10.1016/j.physbeh.2007.04.027. [DOI] [PubMed] [Google Scholar]
- 20.Blazer T, Latzer Y, Nagler RM. Salivary and gustatory alterations among bulimia nervosa patients. European Journal of Clinical Nutrition. 2008;62:922. doi: 10.1038/sj.ejcn.1602801. [DOI] [PubMed] [Google Scholar]
- 21.Dynesen AW, Bardow A, Astrup A, Petersson B, Holst JJ, Nauntofte B. Meal-induced compositional changes in blood and saliva in persons with bulimia nervosa. American Journal of Clkinical Nutrition. 2008;87:12–22. doi: 10.1093/ajcn/87.1.12. [DOI] [PubMed] [Google Scholar]
- 22.Franko DL, Wolfe BE, Jimerson DC. Elevated sweet taste pleasantness ratings in bulimia nervosa. Physiol Behav. 1994;56:969–973. doi: 10.1016/0031-9384(94)90331-x. [DOI] [PubMed] [Google Scholar]
- 23.Drewnowski A, Bellisle F, Aimez P, Remy B. Taste and bulimia. Physiol Behav. 1987;41:621–626. doi: 10.1016/0031-9384(87)90320-9. [DOI] [PubMed] [Google Scholar]
- 24.Bartoshuk LM, Duffy VB, Hayes JE, Snyder DJ. Psychophysics of sweet and fat perception in obesity: problems, solutions and new perspectives. Philos Trans R Soc Lond B Biol Sci. 2006;361:1137–1148. doi: 10.1098/rstb.2006.1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bartoshuk LM, Fast K, Snyder DJ. Differences in Our Sensory Worlds: Invalid Comparisons with Labeled Scales. Current Directions in Psychological Science. 2005;14:122–125. [Google Scholar]
- 26.Berridge KC. Food reward: brain substrates of wanting and liking. Neurosci Biobehav Rev. 1996;20:1–25. doi: 10.1016/0149-7634(95)00033-b. [DOI] [PubMed] [Google Scholar]
- 27.Berridge KC. Motivation concepts in behavioral neuroscience. Physiol Behav. 2004;81:179–209. doi: 10.1016/j.physbeh.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 28.Torregrossa AM, Davis JD, Smith GP. Orosensory stimulation is sufficient and postingestive negative feedback is not necessary for neuropeptide Y to increase sucrose intake. Physiol Behav. 2006;87:773–780. doi: 10.1016/j.physbeh.2006.01.018. [DOI] [PubMed] [Google Scholar]
- 29.Hajnal A, Smith GP, Norgren R. Oral sucrose stimulation increases accumbens dopamine in the rat. Am J Physiol Regul Integr Comp Physiol. 2004;286:R31–R37. doi: 10.1152/ajpregu.00282.2003. [DOI] [PubMed] [Google Scholar]
- 30.Casper RC, Sullivan EL, Tecott L. Relevance of animal models to human eating disorders and obesity. Psychopharmacology. 2008 Aug;199(3):313–29. doi: 10.1007/s00213-008-1102-2. [DOI] [PubMed] [Google Scholar]