Figure 5.
Tat inhibits the SIRT1 deacetylase activity
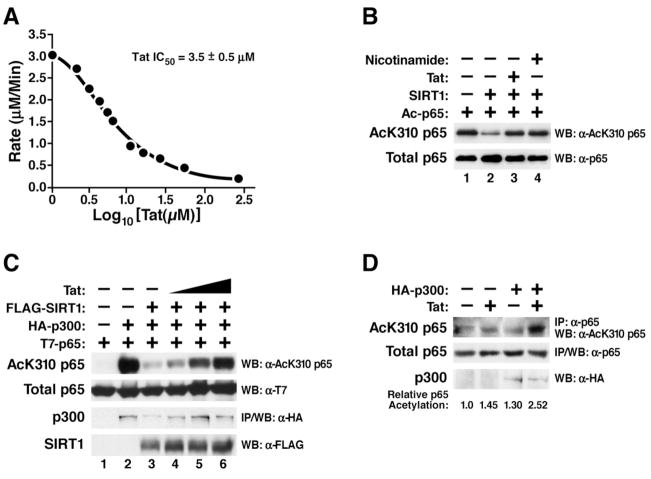
(A) In vitro deacetylation assay of recombinant SIRT1 in the presence of synthetic Tat (aa 1–72). The rate of 1 μM SIRT1 was calculated for 200 μM fluorogenic deacetylase substrate (aa 379–382 of p53) in the presence of twelve concentrations of synthetic Tat. The data were plotted in Prism with X equal to Log10[Tat(μM)] and Y equal to rate. A nonlinear regression was done using the one-site competitive binding equation to determine the IC50.
(B) In vitro deacetylase assay of recombinant SIRT1 and recombinant acetylated p65 in the presence of synthetic Tat or nicotinamide. Western blotting was performed with α-AcK310 p65 and α-p65 antibodies.
(C) In vivo acetylation assay of overexpressed p65. Expression vectors encoding T7-tagged p65 (0.5 μg), p300 (2 μg), SIRT1 (1 μg), and Tat (0.1, 0.25, and 0.5 μg) were transiently cotransfected in 293 cells as indicated. Total cell lysates were analyzed by western blotting with α-AcK310 p65, α-T7, and α-FLAG antibodies. p300 was immunoprecipitated and subjected to western blotting with α-HA antibody.
(D) In vivo acetylation assay of endogenous p65. 293 cells were cotransfected with expression vectors encoding Tat (0.5 μg) and p300 (10 μg) as indicated and treated with TNFα (20 ng/ml) and trichostatin (TSA; 400 nM) over night. TSA is an inhibitor of class I and II HDACs, but not sirtuins and was added to prolong nuclear retention of p65 through hyperacetylation of K218 and K220. Endogenous p65 was immunoprecipitated using α-p65 antibody and subjected to western blotting with α-AcK310 p65 or α-p65 antibodies. Western blotting for p300 was performed with α-HA antibody.