Abstract
Loss of cardiac mitochondrial function with age may cause increased cardiomyocyte death through mitochondria-mediated release of apoptogenic factors. We investigated ventricular subsarcolemmal (SSM) and interfibrillar (IFM) mitochondrial bioenergetics and susceptibility towards Ca2+-induced permeability transition pore (mPTP) opening with aging and lifelong calorie restriction (CR). Cardiac mitochondria were isolated from 8, 18, 29 and 37-month-old male Fischer 344 × Brown Norway rats fed either ad libitum (AL) or 40% calorie restricted diets. With age, H2O2 generation did not increase and oxygen consumption did not significantly decrease in either SSM or IFM. Strikingly, IFM displayed an increased susceptibility towards mPTP opening during senescence. In contrast, Ca2+ retention capacity of SSM was not affected by age, but SSM tolerated much less Ca2+ than IFM. Only modest age-dependent increases in cytosolic caspase activities and cytochrome c levels were observed and were not affected by CR. Levels of putative mPTP-modulating components: cyclophilin-D, the adenine nucleotide translocase (ANT), and the voltage-dependent ion channel (VDAC) were not affected by aging or CR. In summary, the age-related reduction of Ca2+ retention capacity in IFM may explain the increased susceptibility to stress-induced cell death in the aged myocardium.
Keywords: heart disease, ischemia-reperfusion, senescence, hypertrophy
1. Introduction
Aging of the heart encompasses increased cardiomyocyte reactive hypertrophy (Olivetti et al, 2000), cell death by apoptosis (Kajstura et al, 1996; Crow et al, 2004) and necrosis (Olivetti et al, 2000), and infiltration of fibrotic cells (Hacker et al, 2006) with subsequent organ enlargement and weakening (Pugh and Wei, 2001). Cellular and organelle damage inflicted by reactive oxygen species (ROS) (Harman, 2003) can cause loss of molecular fidelity (Hayflick, 2007) resulting in dysfunction (Lesnefsky et al, 2001b) that eventually may activate cell death pathways. Consequently, cardiac function declines with age (Pugh and Wei, 2001), as does the heart's ability to tolerate stress (Misare et al, 1992). Mitochondria, presumably the main cellular source of ROS production (Judge and Leeuwenburgh, 2007), exist in heart muscle as two sub-populations with distinct biochemical (Palmer et al, 1977; Palmer et al, 1985; Chemnitius et al, 1993) and structural (Riva et al, 2005) properties: subsarcolemmal mitochondria (SSM), located beneath the plasma membrane, and interfibrillar mitochondria (IFM) which are found in parallel rows between the myofibrils (Palmer et al, 1977). ROS are produced mainly at respiratory complexes I and III in the form of the superoxide anion (O2•-) that rapidly converts into hydrogen peroxide (H2O2) (Boveris and Chance, 1973). The mitochondrial ‘vicious cycle’ theory of aging implies ROS production during respiration with associated increased oxidative damages leading to mitochondrial dysfunction and further increased ROS production (Hiona and Leeuwenburgh, 2008). However, it is not clear whether aged mitochondria, which are thought to be continuously turned over by mitochondrial biogenesis and autophagy (Menzies and Gold, 1971), lose their function and models of high mtDNA mutational rates (e.g. POLG mice) do not display increased ROS production or oxidative damages (Kujoth et al, 2005).
Mitochondrial membrane permeabilization (MMP) is a central event in apoptosis which can be mediated through mitochondrial permeability transition pore (mPTP) opening, which therefore may play a causal role in myocardial pathology with aging (Di Lisa and Bernardi, 2005; Grimm and Brdiczka, 2007). The pore is thought to exist as a multiprotein complex, currently of unknown composition (Leung and Halestrap, 2008), that spans both the outer and inner mitochondrial membranes. Knockout studies have confirmed a role for cyclophilin D (CyP-D) in pore opening whereas a regulatory role has been ascribed to the adenine nucleotide translocator (ANT) (Leung and Halestrap, 2008). Involvement of the voltage-dependent anion channel (VDAC) was recently ruled out (Baines et al, 2007). Conditions including high calcium (Ca2+) and oxidative stress trigger mPTP opening, causing a sudden influx of small molecules including H2O, resulting in collapse of the membrane potential, mitochondrial swelling and rupture of the outer membrane, followed by the release of pro-apoptotic proteins (i.e., cytochrome c, apoptosis inducing factor and endonuclease G) residing in the intermembrane space (Kroemer et al, 2007). Released cytochrome c complexes with apoptosis protein activator factor-1 (Apaf-1), dATP and procaspase-9 and forms an apoptosome, which then activates caspase-9 (Kroemer et al, 2007). This latter, in turn, cleaves and activates caspase-3, -6, and -7, main executioners of cell death (Kroemer et al, 2007). Ischemic-reperfusion injury, a common pathological event in the aged heart following coronary thrombosis, involves accumulation of lactic acid, drop of ATP and increases in intracellular and mitochondrial Ca2+ levels (Crow et al, 2004; Halestrap, 2006). During the subsequent reperfusion phase, oxidative stress resulting by increased ROS production may determine opening of the mPTP. This series of events has been associated with the execution of both apoptotic and necrotic cell death depending on the length of pore opening (Grimm and Brdiczka, 2007). The matrix oriented CyP-D has been suggested to be the key promoter of mPTP opening in response to Ca2+ overload (Crompton, 2004; Di Lisa and Bernardi, 2006), and opening of the mPTP can be inhibited by the immunosuppressant cyclosporine A through its binding to CyP-D (Halestrap, 2006). Indeed, use of cyclosporin A or similar drugs (e.g., sanglifehrin-A) immediately following reperfusion can limit infarct size and protect myocytes from oxidative stress (Hausenloy et al, 2003).
We recently observed that calorie restriction (CR), an intervention that extends mean and maximum lifespan in short-lived higher organisms, enhanced cardiac autophagy in aged F344 rats (Wohlgemuth et al, 2007), a process becoming defective with age when lysosomes accumulate lipofuscin (Muscari et al, 1990). CR has also been shown to protect from atherosclerosis (Fontana et al, 2004) and attenuate the age-associated decline in heart diastolic function (Taffet et al, 1997; Meyer et al, 2006). However, effects of CR on heart mitochondrial bioenergetics and oxidant generation are still debated and the literature also varies as to whether mitochondria of ad libitum (AL) fed rats suffer respiratory losses as well as if H2O2 production increases with age. Although reports of increased H2O2 production rates with age and decreased rates with CR dominate (Judge and Leeuwenburgh, 2007), also unchanged rates with age (Hansford et al, 1997; Gredilla et al, 2001) and lifelong CR (Drew et al, 2003) have been reported.
In the present study we investigated the effects of aging and lifelong CR on oxygen consumption, H2O2 generation and susceptibility towards mPTP opening in rat ventricular mitochondrial sub-populations. Furthermore, we investigated whether advancing age was associated with changes in levels of mPTP-modulating components and increased cytosolic apoptogenic factors.
2. Materials and methods
2.1. Animals and study design
Male Fischer 344 × Brown Norway hybrid (F344×BN F1) rats were purchased from the National Institute on Aging colony at Harlan Industries (Indianapolis, IN). This strain has increased longevity (median and maximal lifespans is 33.3 and 40.0 months respectively for males, extended to 40.2 and 49.2 months respectively with 40% CR) (Sprott et al. 1996), lower incidence of major pathologic processes than F344 or Wistar rats (Lipman et al, 1996), and age-related body composition changes (i.e. increase in adiposity and decrease in lean mass) that resemble those occurring in humans. The ages were chosen from published growth and survival curves (Sprott and Austad 1996) to reflect a young age (8 mo), adulthood (18 mo), advanced age (29 mo) and senescence (37 mo) at sacrifice. In each age group, 9 animals were fed ad libitum (AL) and 9 were 40% calorie restricted (CR). CR had been initiated at 14 weeks of age at 10% restriction, increased to 25% at 15 weeks, and to 40% at 16 weeks. Rats were received and housed for 4-7 weeks prior date of sacrifice. Rats were individually housed and maintained on a 12-h light/dark cycle under controlled conditions. Health status, body weight and food intake were monitored daily. AL rats had free access to NIH-31 average nutrient composition pellets whereas CR animals received restricted amounts (40% CR) of NIH-31/NIA Fortified pellets (both pellet types were from Harlan Laboratories, Indianapolis, IN) according to tables provided by NIA once daily, 1 h before the onset of the dark period. All rats had free access to tap water. Principles of laboratory animal care (NIH publication No. 86-23, revised 1985) were followed and all procedures were approved by the University of Florida's animal care and use committee.
2.2. Preparation of subcellular fractions
Two randomly chosen rats from different groups were sacrificed daily by rapid decapitation, about 2-3 hours into the light period (CR rats had been fasted for at least 12 h, whereas AL rats were not fasted) over the course of 36 days. The heart was carefully removed, rinsed with saline solution and dissected. After removal of the atria, left and right ventricles were minced together on ice in 5 ml ice-cold homogenization buffer (100 mM KCl, 50 mM MOPS, 5 mM MgSO4, 1 mM ATP, 1 mM EGTA, and 0.4% fatty acid-free BSA, pH 7.4), and homogenized on ice in 10 ml buffer per gram of tissue, using five full strokes with a mechanically driven Potter-Elvehjem glass-teflon homogenizer. Homogenates were centrifuged at 800 × g for 10 min at 4°C to pellet large organelles and myofibrils containing the IFM. The supernatant containing SSM was filtered through synthetic cheese cloth and centrifuged at 8,000 × g for 10 min at 4°C. The resulting supernatant, representing the mitochondria-free cytosolic fraction, was decanted and stored at -80°C, while the pellet, containing SSM was resuspended in 1 ml homogenization buffer without BSA. The initial pellet containing myofibrils (and IFM) was immediately resuspended in 10 ml homogenization buffer (with 0.4% FAF-BSA) per gram tissue and IFM were enzymatically liberated using freshly prepared nagarse (Sigma-Aldrich, St. Louis, MO) at 8 U/g tissue for 1 min under agitation on ice. After five strokes of homogenization, samples were centrifuged at 800 × g for 10 min at 4°C. The supernatant containing IFM was filtered through cheese cloth and centrifuged at 8,000 × g for 10 min at 4°C, the supernatant discarded, and IFM immediately resuspended in homogenization buffer without BSA. Finally, SSM and IFM were washed to remove residual BSA by centrifugation at 8,000 × g for 10 min at 4°C and resuspended in BSA-free buffer. Protein concentration was determined using the method developed by Bradford (Bradford, 1976). The cytosol and remaining mitochondria were frozen in liquid nitrogen for later analysis.
2.3. Electron microscopy
Mitochondria were immersed in Trumps fixative (pH 7.25) over night at 4°C (McDowell and Trump, 1976). The fixed mitochondrial pellet was processed by the following steps with the use of a Pelco Biowave biological microwave oven (Ted Pell Inc., Redding Ca). After removal of Trumps solution, the pellet was washed in 0.1 M sodium cacodylate buffer, encapsulated in 3% agarose, buffer washed and post-fixed in 1% osmium tetroxide. Subsequently, the pellet was dehydrated in a graded ethanol series followed by 100% acetone and embedded in Embed812/Araldite resin (Electron Microscopy Sciences, Hatfield, PA). The epoxy-cured pellet was then mounted, trimmed, sectioned (70-80 nm) and examined on a Hitachi H-7000 transmission electron microscope (Hitachi High Technologies America, Pleasanton, CA). Digital images were acquired by MegaView III (Soft-Imaging Systems, Lakewood, CO). Contamination of the mitochondrial fractions from other organelles was minimal and most mitochondria were intact and non-hypertrophied.
2.4. Mitochondrial Ca2+ retention capacity measurement
Mitochondria in living cells can accumulate [Ca2+]m in the high μM range and isolated mitochondria rapidly accumulate Ca2+ (Bianchi et al, 2004). We determined the maximal Ca2+ loading capacity of freshly isolated mitochondria until Ca2+ release as described by Ichas et al. (Ichas et al, 1997), adapted to 96-well plate format. Ca2+ uptake and release were monitored using the membrane-impermeable fluorescent probe Calcium Green-5N (excitation 506 / emission 532 nm; Molecular Probes, Eugene, OR). SSM (0.75 mg/ml) and IFM (optimal concentrations were found to be 0.1 mg/ml on glutamate/malate and 0.4 mg/ml on succinate) were incubated with shaking in reaction medium (250 mM sucrose, 10 mM Tris, 10 mM KH2PO4, pH 7.4, final volume 250 μl) at 37°C, with respiratory substrates (5 mM glutamate/2.5 mM malate or 5 mM succinate). Injections of 1.25 nmol CaCl2 were performed every 60 s, and repeated until Ca2+ was released into the medium. Extra-mitochondrial Ca2+ was recorded every 7 s using a Synergy HT multi-detection microplate reader (BioTek Instruments, Vinooski, VT). The mPTP inhibitor Cyclosporine A (1 μM, Sigma), was used as a positive control to delay Ca2+-induced PTP opening (Halestrap, 2006). Results are expressed as the amount Ca2+ per mg mitochondria to trigger Ca2+ release through mPTP opening.
2.5. Mitochondrial H2O2 production
Mitochondrial H2O2 production was assessed as described by Barja (Barja, 2002), adapted to 96-well microplate format. Briefly, homovanillic acid is converted into a fluorescent dimer (312 nm excitation / 420 nm emission) in the presence of H2O2 by horseradish peroxidase. Freshly isolated mitochondria (20 μg of mitochondrial proteins) were incubated with assay buffer (145 mM KCl, 30 mM HEPES, 5 mM KH2PO4, 3 mM MgCl2, 0.1 mM EGTA, 0.3% FAF-BSA, pH 7.4 set at 37°C), followed by the addition of horseradish peroxidase (1.2 U) and homovanillic acid (100 μM). Mitochondria were energized with 2.5 mM glutamate/2.5 mM malate or 5 mM succinate ±2 μM rotenone. Calibration was performed against added commercial H2O2 covering the range produced by the mitochondria. Samples were incubated at 37°C in a light-protected Solo HT microplate incubator (Thermo Electron Co., Milford, MA) placed on an Orbit P4 microplate rotator (Labnet International Inc., Woodbridge, NJ) set at 25 rpm. After 15 min incubation, the reaction was stopped by the addition of ice-cold stop solution (65 μl of 0.1 M glycine-NaOH, 25 mM EDTA, pH 12). The light emission was immediately measured with a SpectraMax Gemini XS microplate fluorometer (Molecular Devices, Sunnyvale, CA). Results are expressed as nmol H2O2/min/mg mitochondrial protein.
2.6. Oxygen consumption measurements
Oxygen consumption of freshly isolated mitochondria was assessed using a Clark-type oxygen electrode (Oxytherm, Hansatech, Norfolk, UK). The respiratory reaction buffer (140 mM KCl, 30 mM HEPES, 5 mM KH2PO4, 0.1 mM EGTA, 0.3% FAF-BSA, pH 7.4) was maintained at 37°C, constantly stirred, and air saturated. IFM or SSM were injected into the chamber (0.25 mg/ml) and state II mitochondrial respiration assessed after injecting glutamate/malate (5/2.5 mM) into the chamber. State III respiration was assessed after injecting ADP (500 μM). To test for outer membrane integrity, cytochrome c (160 μM) was injected during state III respiration and the corresponding oxygen consumption rate measured (Jekabsone et al, 2003). Finally, the uncoupling state was measured after injecting carbonyl cyanide 4-(trifluoromethoxy)-phenylhydrazone (FCCP; 0.88 μM). Rates of oxygen consumption are expressed as nmol/min/mg mitochondrial protein. The respiratory control ratio (RCR) was calculated by dividing the rate of oxygen consumption during state III by that during state II. It should be noted that heart mitochondria normally respire mainly on fatty acids actively imported into the matrix for enzymatic metabolism and oxidation.
2.7. Western blot analysis for components of the mPTP and COX4
Content of ANT, VDAC, CyP-D and subunit IV of the cytochrome c oxidase complex (COX4) was assessed in IFM and SSM by Western blot analysis. Prior to loading, samples were boiled at 95°C for 5 min in Laemmli buffer (62.5 mM Tris-HCl, 2% SDS, 25% glycerol, 0.01% bromophenol blue, pH 6.8; BioRad, Hercules, Ca) with 5% β-mercaptoethanol. Ten μg of mitochondrial proteins were separated using 10% Tris-HCl precast gels (BioRad, Hercules, CA), and transferred to polyvinylidene difloride (PVDF) membranes (Immobilon P, 0.45 μm, Millipore, Billerica, MA) using a semidry blotter (BioRad). Transfer efficiency was verified by staining the gels with GelCode Blue Stain Reagent (Pierce Biotechnology, Rockford, IL) and the membranes with Ponceau S (Sigma). Membranes were blocked in StartingBlock TBS Blocking Buffer with 0.05% Tween-20 (Pierce Biotechnology) for 1 h at room temperature, washed in TBS and incubated overnight with primary antibodies against ANT, VDAC, CyP-D (all 1:1,000; Mitosciences, Eugene, OR) and COX4 (1:1,000; Cell Signaling Technology, Beverly, MA) at 4° C. The next morning, membranes were washed in TBS with 0.05% Tween-20 (TBS-t) and subsequently incubated with alkaline phosphatase-conjugated secondary antibody (Sigma-Aldrich, St. Louis, MO), 1:30,000, at room temperature for 1 h. Membranes were then washed in TBS-t, rinsed in TBS and soaked in Tris·HCl (pH 9.5). Finally, the DuoLux chemiluminescent/fluorescent substrate for alkaline phosphatase from Vector Laboratories (Burlingame, CA) was applied and the chemiluminescent signal captured with an Alpha Innotech Fluorchem SP imager (Alpha Innotech, San Leandro, CA). Digital images were analyzed using AlphaEase FC software (Alpha Innotech). Membranes were subsequently stripped and probed for subunit IV of the cytochrome c oxidase complex (COX4). Spot density of the target band (arbitrary OD units) was normalized to that of the corresponding COX4 band.
2.8. Cytosolic content of cytochrome c
The concentration of cytochrome c in the cytosolic fraction was determined using an enzyme-linked immunosorbent assay (ELISA) kit (R&D Systems, Minneapolis, MN), following the manufacturer's instructions. Absorbance was determined at 450 nm (wavelength correction at 570 nm) using a SpectraMax 340 microplate reader (Molecular Devices, Sunnyvale, CA). Cytochrome c concentration is reported as ng/μg cytosolic protein.
2.9. Caspase -9 and -3 activity
Activities of caspase-9 and -3 were measured in the cytosolic fraction using fluorometric assays (BioVision Research Products, Mountain View, CA) as per the manufactorer's directions. Briefly, 50 μg cytosolic proteins were incubated (1.5 h at 37°C) with caspase-9 or caspase-3 specific substrate (LEHD-AFC and DEVD-AFC, respectively). Cleavage of the substrate frees AFC, which emits at 505 nm (400 nm excitation). Measurements were performed using a SpectraMax Gemini XS microplate fluorometer. Results are expressed as arbitrary emission units.
2.10. Statistics
Differences among experimental groups were analyzed by two-way ANOVA with age and diet as factors, followed by Tukey's multiple comparison test when needed. In addition, two-way ANOVA for repeated measures was used to compare SSM and IFM from the same animals. Correlations between variables were explored using the Pearson's test. Statistical significance was set to p < 0.05, and analyses conducted using GraphPad Prism 4 (San Diego, California). Data are shown as mean ± SEM.
3. Results
3.1. Body and heart weights
CR animals had significantly lower body and heart weights than AL animals (p < 0.001). Body weights were highest at 29 months in both groups and declined thereafter (Table 1). Notably, heart weight increased progressively with age in both AL and CR rats, although the increase was much smaller with CR which counteracted excessive growth. The effect of CR became larger with increasing age both for heart and body weights (interaction effects: p < 0.001, Table 1). CR animals had greater heart-, and left (LV) and right (RV) ventricle-to-body-weight ratios at all ages versus AL animals (p < 0.001) likely due to their lighter bodies, and therefore not indicative of cardiac hypertrophy. In line with the previous observation that LV mass of male F344×BN rats progressively increases in aging (up to 39 months) (Hacker et al, 2006), we observed an increase in both LV and RV mass, although the larger LV displayed a greater increase with age (43%), comparing 37 to 18 months AL rats, than the RV (24% when expressed per body weight).
Table 1.
Body and heart weights for male F344xBN rats
| Age (mo) | AL | CR | ||||||
|---|---|---|---|---|---|---|---|---|
| 8 | 18 | 29 | 37 | 8 | 18 | 29 | 37 | |
| N | 9 | 9 | 9 | 9 | 9 | 9 | 9 | 9 |
| BW (g) | 377±15 | 479±6 | 538±7 | 486±10 | 268±5 | 307±3 | 318±3 | 270±4 |
| Age effect | a,b,c | a,d | b,d,e | c,e | f,k | k,l | f,g | g,l |
| Diet effect | +++ | +++ | +++ | +++ | ||||
| Overall (two-way ANOVA) | p < 0.001 (↑ with age), p < 0.001 (↓ with CR), p < 0.001 (interaction) | |||||||
| Heart (mg with atrium) | 898±29 | 1,075±14 | 1,266±23 | 1,487±28 | 714±10 | 809±13 | 904±17 | 922±14 |
| a,b,c | a,d,e | b,d,f | c,e,f | g,h,k | i,k,l | g,l | h,i | |
| +++ | +++ | +++ | +++ | |||||
| p < 0.001 (↑ with age), p < 0.001 (↓ with CR), p < 0.001 (interaction) | ||||||||
| LV (mg) | 446±16 | 536±22 | 698±25 | 779±25 | 347±8 | 410±14 | 472±21 | 483±20 |
| a,b,k | c,d,k | a,c | b,d | e,f | e | f | ||
| + | +++ | +++ | +++ | |||||
| p < 0.001 (↑ with age), p < 0.001 (↓ with CR), p < 0.001 (interaction) | ||||||||
| RV (mg) | 175±10 | 202±7 | 226±6 | 251±11 | 140±5 | 146±5 | 160±4 | 156±4 |
| a,b | c | a | b,c | |||||
| + | +++ | +++ | +++ | |||||
| p < 0.001 (↑ with age), p < 0.001 (↓ with CR), p < 0.001 (interaction) | ||||||||
| Heart/BW, ×10-3 | 2.39±0.04 | 2.25±0.03 | 2.35±0.02 | 3.07±0.07 | 2.67±0.03 | 2.63±0.04 | 2.84±0.05 | 3.43±0.08 |
| a | b | c | a,b,c | d | e | f | d,e,f | |
| ++ | +++ | +++ | +++ | |||||
| p < 0.001 (↑ with age), p < 0.001 (↑ with CR) | ||||||||
| LV/BW, ×10-3 | 1.19±0.04 | 1.12±0.05 | 1.30±0.04 | 1.60±0.04 | 1.30±0.05 | 1.34±0.05 | 1.49±0.07 | 1.79±0.07 |
| a | b | i | a,b,i | c | d | j | c,d,j | |
| p < 0.001 (↑ with age), p < 0.001 (↑ with CR) | ||||||||
| RV/BW, ×10-3 | 0.46±0.01 | 0.42±0.01 | 0.42±0.01 | 0.52±0.03 | 0.52±0.01 | 0.48±0.02 | 0.50±0.01 | 0.58±0.02 |
| i | j | i,j | a | k | a,k | |||
| + | ||||||||
| p < 0.001 (↑ with age), p < 0.001 (↑ with CR) | ||||||||
Data are given as mean ±SEM with indication of statistical significant results below each row.
Data sharing the same letter in each row are significantly different (a-hp < 0.001, i,jp < 0.01, k,lp < 0.05) by Tukey's multiple comparison test for age within each treatment (diet).
Significant effects of diet at each age by Tukey's multiple comparison test are marked with signs (+++p < 0.001, ++p < 0.01, +p < 0.05) placed under the higher value.
Results from overall two-way ANOVA analyses are shown below.
Abbreviations: AL, ad libitum; CR, calorie restricted; BW, body weight, LV, left ventricle; RV, right ventricle.
3.2. Mitochondrial Ca2+ retention capacity
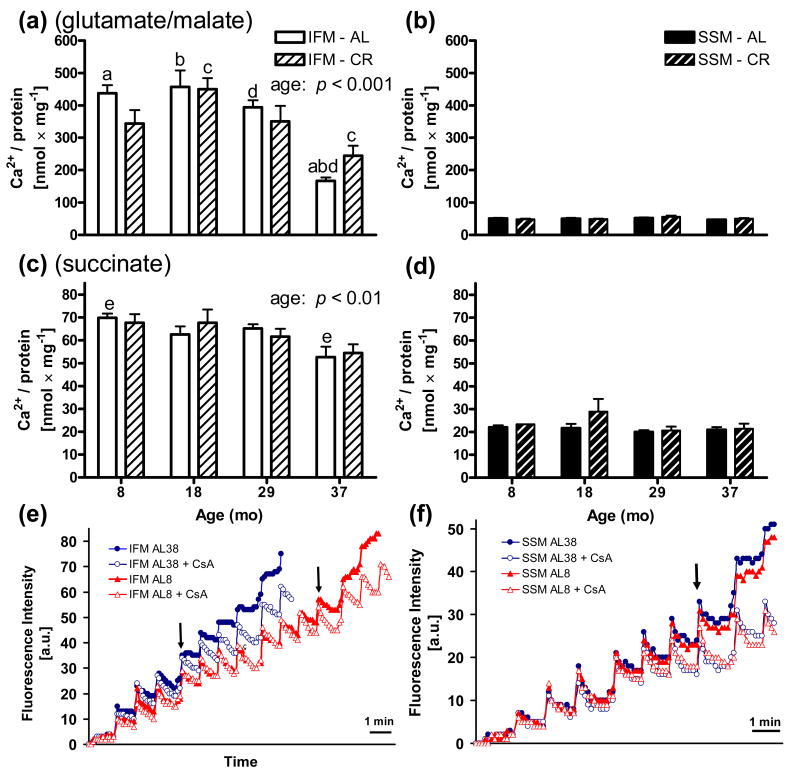
Whereas SSM were unaffected with age on either substrate (Figs. 1b and d), IFM strikingly displayed a decreased Ca2+ retention capacity with increasing age for both respiratory substrates, giving significant main effects for age with glutamate/malate (p < 0.001) and succinate (p < 0.01) (Figs. 1a and c). Co-incubation with Cyclosporine A inhibited mitochondrial burst even after high Ca2+ loads, indicating that mitochondrial membrane permeabilization involved CyP-D. For both substrates, IFM were significantly more resistant to Ca2+-induced mPTP opening than SSM. Both SSM and IFM were significantly more sensitive to Ca2+-induced PTP opening in the presence of succinate, a succinate oxidoreductase (complex II) electron donor, than glutamate/malate, a NADH:ubiquinone oxidoreductase (complex I) electron donor. Whereas CR had no effect on the Ca2+ retention capacity for SSM, CR counteracted mPTP opening at 37 months in IFM energized with glutamate/malate.
Fig. 1.
Ca2+ retention capacities in heart ventricular mitochondria. Pulsed Ca2+ mitochondrial uptake monitored until mPTP opening using glutamate/malate (top figures) or succinate (middle figures) as respiratory substrates. Bars show how much Ca2+ can be added in pulses before mPTP opening. A significant decline in Ca2+ retention capacity was observed with age particularly during senescence for IFM (left side) on either glutamate/malate (p < 0.001) (a) or succinate (p < 0.01) (c) with two-way ANOVA. SSM (right side) displayed no age-effect for either substrate (b, d) and had significantly (p < 0.001-0.05 with two-way repeated measures ANOVA) lower Ca2+ retention capacities than IFM for all groups (not indicated). Use of the greater ROS producer succinate increased the vulnerability towards mPTP opening compared to glutamate/malate for both sub-populations. SSM were maintained at 0.75 mg/ml and IFM at either 0.1 mg/ml (glutamate/malate) or 0.4 mg/ml (succinate). Co-incubation with the PTP-inhibitor CsA prevented mPTP opening. Complementary analyses by one-way ANOVA with Tukey's post-hoc test gave significant differences between bars sharing the same letter (a,bp < 0.001, c,dp < 0.01, ep < 0.05) within each sub-set of mitochondria. No statistical differences were found between AL and CR groups of the same age. Data are shown as mean ±SEM nmol Ca2+ uptake per mg mitochondrial protein until mPTP opening, triggering a burst of Ca2+ release. Bottom figures show examples of extra-mitochondrial Ca2+ monitoring using the fluorescent probe Calcium Green-5N in (e) IFM (0.1 mg/ml) and (f) SSM (0.75 mg/ml) from 8 and 37 mo old AL fed rats using glutamate/malate as substrates. 1.25 nmol Ca2+ was added every minute. Arrows (↓) indicate when Ca2+ uptake begins to become difficult. The mPTP inhibitor cyclosporin A (CsA) prevented Ca2+-induced burst. Abbreviation: a.u. = arbitrary units.
3.3. H2O2 production
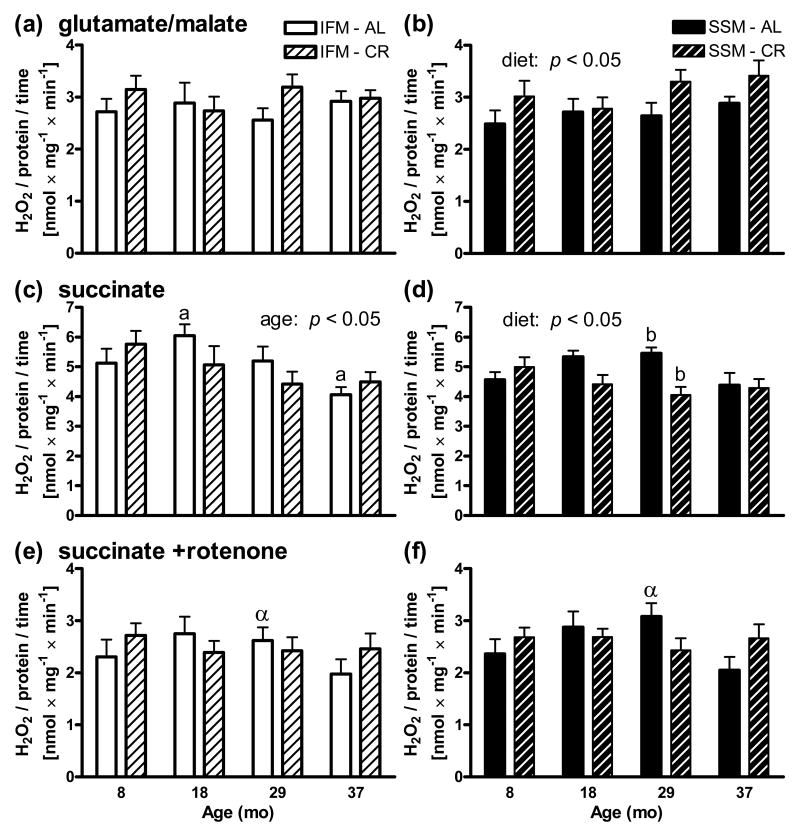
Use of succinate as a respiratory substrate generated higher production of H2O2 than glutamate/malate (Fig. 2). Rotenone, which blocks electron back-flow from succinate dehydrogenase to complex I, reduced H2O2 production back to levels generated by glutamate/malate. For the tested substrates there were no significant differences in H2O2 production between SSM and IFM and no significant increases in H2O2 production with age for any substrate or sub-population were observed. Indeed, for IFM on succinate a significant (p < 0.05) decline in H2O2 production was observed with age (Fig. 2c). For SSM, a slight increased rate (p < 0.05) was observed with CR on glutamate/malate, whereas a slight decreased rate (p < 0.05) was observed with CR on succinate with two-way ANOVA analyses within each sub-set of mitochondria.
Fig. 2.
ROS production in heart mitochondria as measured by H2O2. Similar rates of H2O2 production were observed for IFM (left side) and SSM (right side) for (a,b) glutamate/malate, (c,d) succinate, and (e,f) succinate +rotenone. Production was higher for succinate than glutamate/malate, and addition of rotenone reduced levels back to those of glutamate/malate. For SSM, a slight increased rate (p < 0.05) was observed for CR on glutamate/malate (b), whereas a slight decreased rate (p < 0.05) was observed for CR on succinate (d) with two-way ANOVA analyses within each sub-set of mitochondria. Moreover, two-way ANOVA also detected a slight decrease (p < 0.05) for IFM on succinate with age (c). Significant differences by Tukey's multiple comparison test for all groups within each sub-set of mitochondria share the same letter (a,bp < 0.05), and significant differences between SSM and IFM groups of the same age and diet by two-way repeated measures ANOVA share the same Greek letter (αp < 0.05). Data are expressed as mean ±SEM nmol H2O2 /min and mg mitochondrial protein.
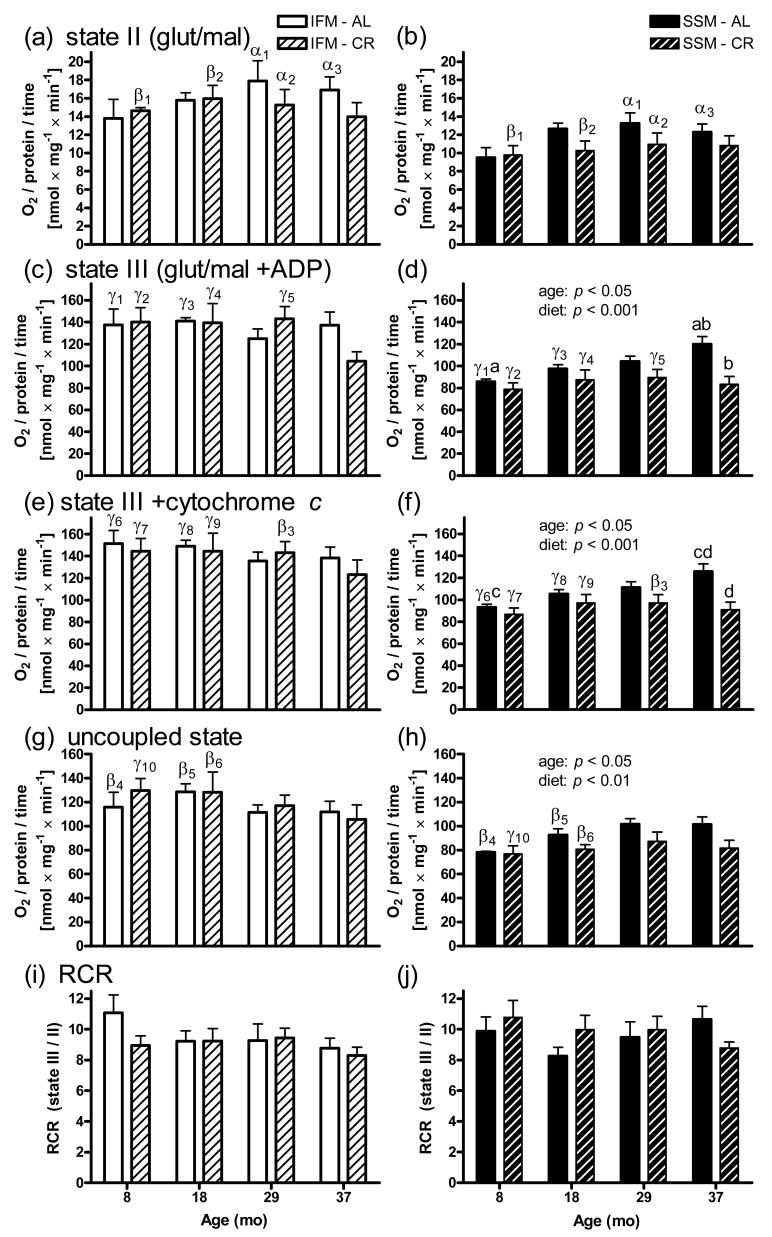
3.4. Oxygen consumption
For states II, III, and when uncoupled, IFM generally consumed more O2 than SSM (Fig. 3). No significant declines with age were observed with two-way ANOVA analyses within each sub-set of mitochondria, although for IFM, a non-significant decrease at 37 months with CR was observed for state III (Fig. 3c). For SSM in two states (III and uncoupled), a slight, yet significant increase (p < 0.05 with two-way ANOVA) in respiration with age was observed particularly for AL fed rats, whereas in CR animals respiration did not change with age, but was significantly lower compared to AL particularly at high age (Figs. 3d and h). Respiration during state III and the uncoupled state were similar. Addition of cytochrome c during state III was performed to test for mitochondrial membrane integrity (Figs. 3e and f). Only a small increase in O2 consumption after cytochrome c addition (less than 10% and evenly distributed) was detected. Therefore, the observed effects were not due to differential mitochondrial deterioration during the isolation procedures.
Fig. 3.
O2 consumption in heart mitochondria. IFM (left side) generally displayed a greater respiratory rate than SSM (right side) for (a,b) state II (glutamate/malate), (c,d) state III (glutamate/malate +ADP), and (g,h) the uncoupled state (glutamate/malate +FCCP), whereas rates were similar for (i,j) RCR (=state III/state II). SSM displayed decreased rates with CR both for state III (p < 0.001) and when uncoupled (p < 0.01) as well as slightly increased rates (p < 0.05) with age for both these states with two-way ANOVA analyses within each sub-set of mitochondria. Test of outer mitochondrial membrane integrity (e,f) by adding exogenous cytochrome c in state III (glutamate/malate +ADP) insignificantly changed the respiratory activity. Significant differences by Tukey's multiple comparison test for all groups within each sub-set of mitochondria share the same letter (a,cp < 0.05, b,dp < 0.01), and significant differences between SSM and IFM groups of the same age and diet by two-way repeated measures ANOVA share the same numbered Greek letter (αp < 0.05, βp < 0.01, γp < 0.001), respectively. Data (except RCR) are expressed as mean ±SEM nmol O2/min and mg mitochondrial protein. Abbreviations: RCR, respiratory control ratio (state III/state II).
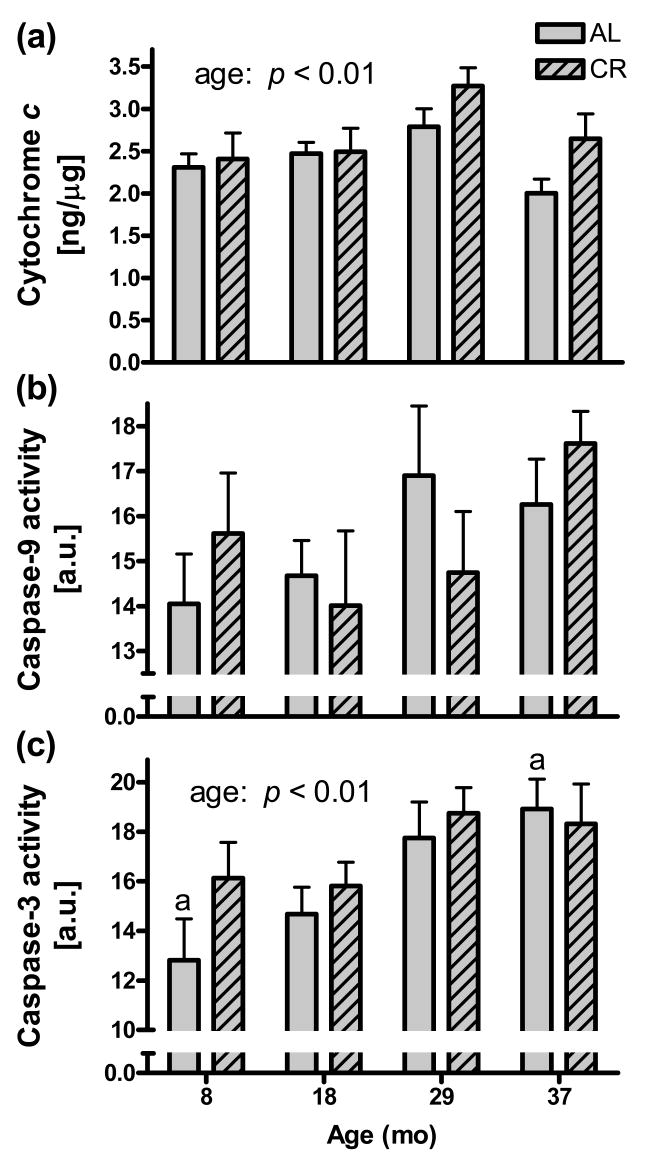
3.5. Cytosolic cytochrome c, and caspase-9 and -3 activities
Cytosolic cytochrome c levels displayed a main age effect (p < 0.01), being highest in the 29 months groups for both AL and CR, after which levels dropped (Fig. 4a). Caspase-9 activity tended to increase with age, but the effect was not significant (p = 0.18) (Fig. 4b). Caspase-3 activity, however, increased significantly with age (p < 0.01) (Fig. 4c). Moreover, caspase-3 activity correlated with the Ca2+ retention capacity for IFM on succinate (r = - 0.30; p < 0.05 for AL and CR animals together, data not shown). Thus, Ca2+ retention capacity was lower in IFM (on succinate) from hearts having higher cytosolic caspase-3 activity (both effects observed in aging).
Fig. 4.
Heart cytosolic cytochrome c levels and caspase-9 and -3 activities. (a) Cytosolic cytochrome c levels increased significantly with age (p < 0.01) up to 29 mo. The concentration is expressed per concentration cytosolic proteins. (b) Cytosolic caspase-9 activities increased with age, but the effect was not significant (p = 0.18). (c) Cytosolic caspase-3 activities increased significantly with age (p < 0.01). The bars sharing the same letter (ap < 0.01) are significantly different with Tukey's. No statistical differences were found between AL and CR groups of the same age.
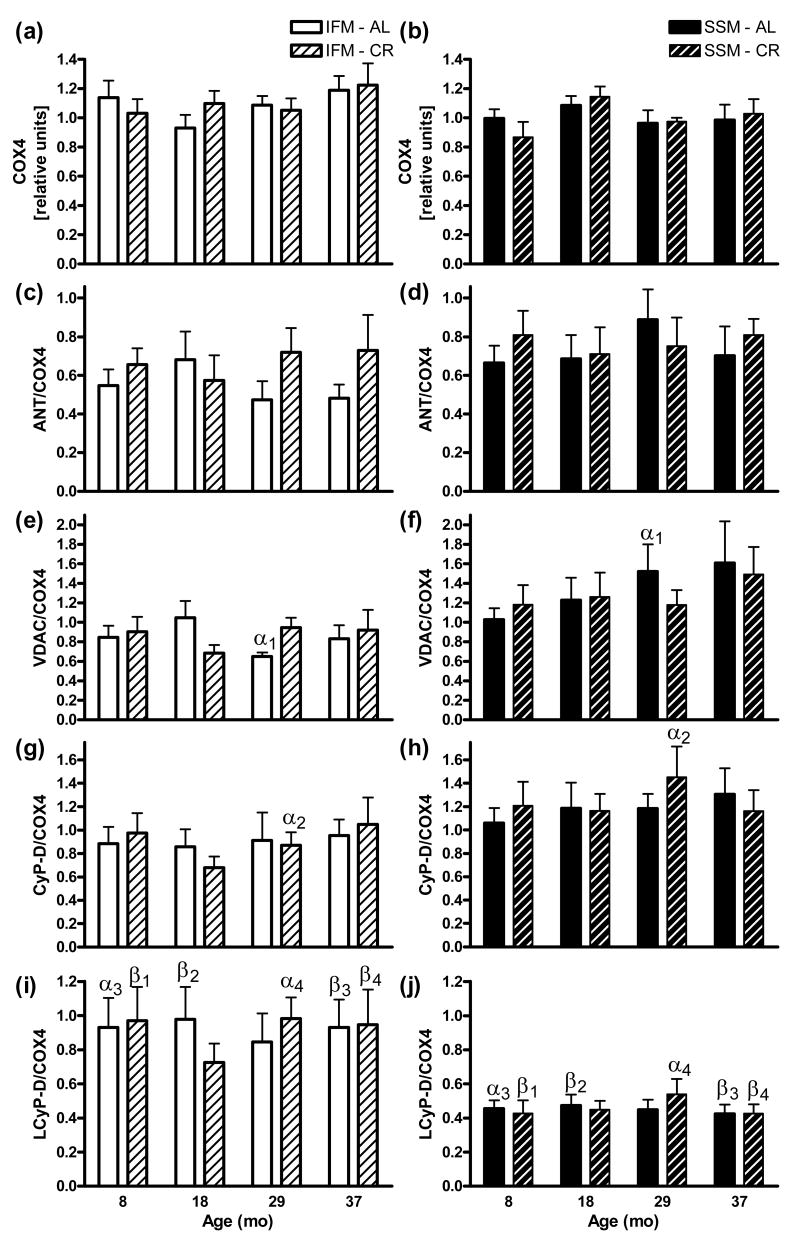
3.6. Western blot analyses of putative mPTP constituents and COX4
Levels of the nuclear encoded respiratory chain subunit COX4 was marginally greater expressed in IFM than SSM, whereas ANT, VDAC, CyP-D were generally greater expressed in SSM than IFM, but no protein changed significantly with age or CR (Fig. 5). Analyses of SSM and IFM in direct comparison using a commercial antibody against CyP-D detected two bands in close proximity. The heavier band (CyP-D, 21 kDa) had higher intensity than the lighter band (here referred to as LCyP-D), with this latter exhibiting higher levels in IFM than in SSM at all ages). LCyP-D was significantly greater expressed in IFM than SSM. Also CyP-D/ANT, CyP-D/VDAC, LCyP-D/ANT, and LCyP-D/VDAC ratios were unaffected by either age or CR (data not shown).
Fig. 5.
Heart mitochondrial protein levels by western blot. COX4 gel band intensities for (a) IFM and (b) SSM were related to that of an identical cross sample (set to 1) run in parallel in all gels, or expressed directly per COX4 of the same sample as for (c,d) ANT, (e,f) VDAC, (g,h) CyP-D (21 kDa) and (I,j) LCyP-D (an ∼18 kDa protein greater expressed in IFM also recognized by the CyP-D antibody). Significant differences between SSM (left) and IFM (right) groups of the same age and diet by two-way repeated measures ANOVA share the same Greek letters (αp < 0.05, βp < 0.01). Two-way ANOVA and Tukey's multiple comparison tests for all groups within each sub-set of mitochondria gave no significant outcomes. Data are expressed as mean ±SEM.
4. Discussion
Results from the present study show that ventricular IFM, but not SSM, become increasingly vulnerable to Ca2+-induced mitochondrial membrane permeabilization during senescence and that cytosolic indicators of apoptosis (caspases and cytochrome c) slightly increase with age. These findings may relate to the observed increased extent of apoptosis in aged hearts (Kajstura et al, 1996; Olivetti et al, 2000). Particularly, our data may help explain the greater susceptibility to ischemia-reperfusion damage observed in the aged hearts (Pepe, 2000; Halestrap, 2006). Our results also confirm that SSM and IFM are bioenergetically and compositionally different.
Apart from that IFM became increasingly susceptible to Ca2+-induced mPTP opening with age, also a drastic difference in Ca2+-induced mPTP opening susceptibility between SSM (highly susceptible) and IFM (less susceptible) was observed (Fig. 1). Moreover, mPTP opening susceptibility was affected by the respiratory substrate used. It has been suggested that mPTP opening is assisted by ROS (Pepe, 2000; Halestrap, 2006), and indeed, use of the respiratory complex II electron donor succinate, which causes enhanced ROS production due to electron back-flow generating superoxide (O2•-), significantly lowered Ca2+ retention capacity compared to the complex I donor glutamate/malate (Fig. 1), However, mitochondrial ROS production was likely not responsible for the changes in mPTP opening susceptibility as H2O2 production did not increase with age and was similar between sub-populations. CR counteracted the age-related loss in Ca2+ retention capacity at 37 months when using glutamate/malate (low ROS producer), but not when using succinate (high ROS producer).
We then investigated if differences in mitochondrial Ca2+ sensitivity with age and between IFM and SSM could be linked to altered expression of mPTP complex proteins. The inhibition of mPTP opening by CsA supports a Ca2+-induced mitochondrial burst mechanism involving CyP-D. However, as levels of CyP-D, ANT and VDAC did not change among the groups, the increased Ca2+ sensitivity may be more related to physical changes (e.g. membrane damage, or else) with age rather than to protein composition. Another possibility is that defective autophagy with senescence particularly affects turnover of the interfibrill-attached IFM, resulting in longer half-lives of IFM with certain impaired functions. It is also possible that basal mitochondrial Ca2+-levels, where high Ca2+ activates key enzymes involved in substrate oxidation (Hansford and Zorov, 1998), are higher in senescent IFM to compensate for eventual functional declines, maintaining respiratory levels but consequently lowering additional Ca2+ uptake capacity. In this study, two bands in close proximity were detected when analyzing CyP-D immunoblots. Interestingly and in support of our data, Tanveer et al. 1996 identified two rat liver mitochondrial peptidylprolyl cis-trans isomerases (PPIase; a property of CyPs) of 18 and 21 kDa using cyclosporin A photolabeling. The larger (identified to be CyP-D) dominated and resided in the matrix, whereas the 18-kDa PPIase located to the intermembrane space (Tanveer et al, 1996). The CyP-D antibody used in our study appears to have detected these two proteins (masses were similar to those reported by Tanveer). Interestingly, the heavier form dominated also in rat cardiac mitochondria, and we found that the lighter form was significantly more expressed in IFM than in SSM (Fig. 5). However, it is unclear whether this different pattern of expression has functional implications.
Apoptosis in postmitotic tissues such as the heart can be expected to be a slowly progressing event being difficult to detect at any given instant. In this context, the slightly increasing cytosolic cytochrome c levels up to 29 months (Fig. 4a) indicates increased ventricular mitochondrial membrane permeabilization events with age possibly due to mPTP opening, Bax permebilization or else (Kroemer et al, 2007). It is unclear why the cytochrome c levels dropped at 37 mo, but this could be related to increased number of young cells due to fibrosis (Hacker et al, 2006) or myocyte plasticity (Ellison et al, 2007) as the ventricles substantially gained mass at senescent age (Table 1). However, the modest insignificant increase in cytosolic caspase-9 activity did not correlate with cytosolic cytochrome c levels. Nevertheless, the activity of the final executioner caspase-3 (Fig. 4c) was highest at the highest ages (29 and 37 months). This may suggests that other apoptotic pathways, including caspase-8 activation by death receptor ligation (Gill et al, 2002), might have contributed to the downstream caspase-3 activation. Our group previously reported increased cytochrome c levels with age in F344 rat heart cytosols in a study spanning up to 24 months (Phaneuf and Leeuwenburgh, 2002), but whether apoptosis or necrosis dominate in age-related cardiomyocyte death is presently still unclear (Olivetti et al, 2000).
We observed no increased H2O2 production with age in, or between, the two different sub-populations for either of the employed substrates, and mitochondrial production rates from CR animals were similar to AL controls. However, also a constant level of H2O2 migrating from mitochondria or stemming from other sources (e.g. enzymatic metabolism and inflammation) will lead to oxidative damages accumulating with advancing age (Harman, 2003). Although both H2O2 production and Ca2+-induced mPTP opening were substrate dependent, H2O2 was likely not causal to the observed differences in Ca2+ retention capacity with age or sub-population. The observed mitochondrial H2O2 production rates are similar to those previously reported (Judge and Leeuwenburgh, 2007) although reported levels vary considerably, which could be due to different procedures used (degree of agitation, temperature, use of microplate or cuvettes having different surface/volume ratios, or mode of calibration: against glucose oxidase produced or added H2O2 as here). Reports also vary in their conclusions as to whether H2O2 production increases with aging, although evidence therefore dominates (Judge and Leeuwenburgh, 2007). In support to our data, a previous study found unchanged H2O2 production with age when analyzing total (mixed types) cardiac mitochondria from senescent (24 mo old) relative to young (6 mo) male rat hearts (Hansford et al, 1997), and similarly, no significant differences in aging were found comparing total mixed type mitochondria from 7 and 24 mo old rat hearts (Gredilla et al, 2001). A third study found that total mitochondria isolated from old (23 mo) rat hearts generated more hydrogen peroxide (H2O2) than did mitochondria from young (3 mo) rats (Nohl and Hegner, 1978), and a fourth that rat heart SSM produced more H2O2 at 14, 18 and 24 mo with comparison to 3 mo (Muscari et al, 1990). However, the use of 3 month-old rats can be questioned as animals may not be fully developed, and for the latter study there was no further increase in H2O2 production after 14 mo, rather a decrease at 18 and 24 mo of age (Muscari et al, 1990). When sub-populations were separated, one study found that whereas the ROS production was similar for SSM and IFM in young (2-5 mo) rats, IFM from old (24-28 mo) rat hearts produced significantly more ROS, but no significant increase was found for SSM with age (Suh et al, 2003). Our group previously observed greater H2O2 production rates for cardiac SSM from 24 than 6 mo F344 hearts, whereas for IFM the age-effect was not significant (Judge et al, 2005a). In the same study, we also observed less H2O2 production from IFM than SSM at 24 mo (Judge et al, 2005a). It is possible that the F344xBN strain is less affected with age, as was shown in this study.
Very few reports are available concerning the effects of lifelong CR on heart mitochondrial H2O2 production. One study observed that CR (40% for 12 mo) initiated at 12 mo significantly lowered rat heart mixed mitochondrial H2O2 production at 24 mo of age with pyruvate/malate, but not succinate, as the respiratory substrates (Gredilla et al, 2001). The same group later found that CR for 4 mo or shorter had no significant effect on H2O2 production (Gredilla et al, 2002). A more recent study found that 3 months of 40% CR reduced cardiac H2O2 production in mid-life (15 mo) rats, but the study did not concern age-related changes (Colom et al, 2007). Our group previously found no significant change in H2O2 production comparing 12 and 26 mo of age, and no effect was seen at 26 mo with 40% life-long CR, analyzing Fischer 344 rat heart SSM (Drew et al, 2003). In contrast, we did find a small (14%) decrease in SSM H2O2 production with short-term (2 mo) CR in 6 mo male F344 rats (Judge et al, 2004). CR affects neuroendocrine and metabolic pathways, alters sirtuin protein expression etc, but if and how these changes affects mitochondrial H2O2 production and in which tissues, is still unclear.
For respiratory measurements we obtained highly satisfactory RCR values (Fig. 3), and in our study and in the early reports by Palmer (Palmer et al, 1977; Palmer et al, 1985), the O2 consumption was lower in SSM than IFM. COX4 levels were marginally lower in SSM, and for either sub-population no significant changes with age or CR were found (Fig. 5). Cardiac mitochondrial respiration in aging has commonly been investigated, although most studies have concerned total mitochondria or only SSM. In a few recent studies covering both sub-populations, however, a tendency toward state III respiration decline with age in rat cardiac IFM was observed (Fannin et al, 1999; Lesnefsky et al, 2001a; Judge et al, 2005b), but not in SSM (Fannin et al, 1999; Lesnefsky et al, 2001a; Cocco et al, 2005; Judge et al, 2005b). When analyzing total mitochondria, one study found no evidence for a significant decrease in respiratory activities with age (Manzelmann and Harmon, 1987), and another reported no change for state II, but a decrease in state III respiration with age (Jahangir et al, 2001). A study concerning human heart (also mixed types of mitochondria) found no decline in respiratory chain activities (Miro et al, 2000). However, also increased respiration with age has been reported. One report on rat SSM found that the respiratory control ratio was unchanged at the ages 3, 14, 18 mo, but increased at 24 mo (Muscari et al, 1990). Another study analyzing SSM from 9, 17 and 23 mo old mouse hearts reported increased state IV respiration with age and decreased respiration with lifelong 40% CR (Sohal et al, 1994). The O2 consumption was unrelated to the decline in IFM Ca2+ retention capacity with age, which suggests that these parameters are not directly related and that respiratory measurements alone may be insufficient for determination of the functionality of mitochondria. Related to the observed unchanged levels of respiration and COX4 with age, one study reported unchanged cardiac mitochondrial cytochrome contents in aging (Manzelmann and Harmon, 1987), and another found unchanged levels of COX1 and COX3 mRNA transcripts as well as COX activity in aging (Barazzoni et al, 2000). However, also decreased levels of COX have been reported (Muller-Hocker, 1989).
Alternatively to altered expression of mPTP constituting proteins or mitochondrial H2O2 production rates in aging, mPTP opening susceptibility could be related to alterations of the lipid components of the inner mitochondrial membrane (Miro et al, 2000; Judge and Leeuwenburgh, 2007), especially of cardiolipin (cardiolipin oxidation releases cytochrome c) (Petrosillo et al, 2001; Di Lisa and Bernardi, 2005), and/or that background Ca2+ or free iron levels (mitochondria handle large amounts of iron) differ. It has also been suggested that Ca2+ binding to cardiolipin may trigger ANT to form a non-specific outer membrane channel, by releasing positive charges within ANT which opens the gate (Brustovetsky and Klingenberg, 1996; Crompton, 2004). However, both decreased (Pepe et al, 1999) and unchanged (Moghaddas et al, 2002) levels of heart cardiolipin with aging have been reported. Thus, the reason for the age-related mPTP opening phenomena observed in IFM needs to be further investigated.
In summary, the data indicate that IFM in senescent hearts are more susceptible to stressful Ca2+-loads once they arise (during ischemia-reperfusion, conditions involving strong contractions, or else) then in adult or young, which can trigger mPTP opening with release of proapoptotic inducing factors. More knowledge regarding the molecular changes in aging causing IFM to lose Ca2+ retention capacity during senescence is of prime interest and warrants further investigation. Also, the contribution of apoptotic pathways and necrosis to cardiomyocyte death with age warrants direct comparisons. The long-lived F344×BN strain displayed no increase in cardiac mitochondrial H2O2 release with age which could relate to this strain's long lifespan. Further to this point, as also respiration was not significantly changed at senescence, advantageous effects due to CR could not be seen on these parameters in this strain. Still, it remains possible that present methods do not pick up subtle changes in mitochondrial respiration or H2O2 production occurring in vivo.
Supplementary Material
Acknowledgments
We thank Karen Kelley at the Electron Microscopy unit at the University of Florida for taking supporting TEM pictures.
This research was supported by grants from the National Institute on Aging (AG17994 and AG21042) to CL, an American Heart Postdoctoral Fellowship to TH (0525346B), and an American Heart Fellowship to AYS (0615256B). EM and SEW are supported by the Claude D. Pepper Older Americans Independence Center (OAIC) (1 P30 AG028740-01).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Baines CP, Kaiser RA, Sheiko T, Craigen WJ, Molkentin JD. Voltage-dependent anion channels are dispensable for mitochondrial-dependent cell death. Nat Cell Biol. 2007;9:550–555. doi: 10.1038/ncb1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barazzoni R, Short KR, Nair KS. Effects of aging on mitochondrial DNA copy number and cytochrome c oxidase gene expression in rat skeletal muscle, liver, and heart. J Biol Chem. 2000;275:3343–3347. doi: 10.1074/jbc.275.5.3343. [DOI] [PubMed] [Google Scholar]
- Barja G. The quantitative measurement of H2O2 generation in isolated mitochondria. J Bioenerg Biomembr. 2002;34:227–233. doi: 10.1023/a:1016039604958. [DOI] [PubMed] [Google Scholar]
- Bianchi K, Rimessi A, Prandini A, Szabadkai G, Rizzuto R. Calcium and mitochondria: mechanisms and functions of a troubled relationship. Biochim Biophys Acta. 2004;1742:119–131. doi: 10.1016/j.bbamcr.2004.09.015. [DOI] [PubMed] [Google Scholar]
- Boveris A, Chance B. The mitochondrial generation of hydrogen peroxide. General properties and effect of hyperbaric oxygen. Biochem J. 1973;134:707–716. doi: 10.1042/bj1340707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Brustovetsky N, Klingenberg M. Mitochondrial ADP/ATP carrier can be reversibly converted into a large channel by Ca2+ Biochemistry. 1996;35:8483–8488. doi: 10.1021/bi960833v. [DOI] [PubMed] [Google Scholar]
- Chemnitius JM, Manglitz T, Kloeppel M, Doenst T, Schwartz P, Kreuzer H, Zech R. Rapid preparation of subsarcolemmal and interfibrillar mitochondrial subpopulations from cardiac muscle. Int J Biochem. 1993;25:589–596. doi: 10.1016/0020-711x(93)90668-5. [DOI] [PubMed] [Google Scholar]
- Cocco T, Sgobbo P, Clemente M, Lopriore B, Grattagliano I, Di PM, Villani G. Tissue-specific changes of mitochondrial functions in aged rats: effect of a long-term dietary treatment with N-acetylcysteine. Free Radic Biol Med. 2005;38:796–805. doi: 10.1016/j.freeradbiomed.2004.11.034. [DOI] [PubMed] [Google Scholar]
- Colom B, Oliver J, Roca P, Garcia-Palmer FJ. Caloric restriction and gender modulate cardiac muscle mitochondrial H2O2 production and oxidative damage. Cardiovasc Res. 2007;74:456–465. doi: 10.1016/j.cardiores.2007.02.001. [DOI] [PubMed] [Google Scholar]
- Crompton M. Mitochondria and aging: a role for the permeability transition? Aging Cell. 2004;3:3–6. doi: 10.1046/j.1474-9728.2003.00073.x. [DOI] [PubMed] [Google Scholar]
- Crow MT, Mani K, Nam YJ, Kitsis RN. The mitochondrial death pathway and cardiac myocyte apoptosis. Circ Res. 2004;95:957–970. doi: 10.1161/01.RES.0000148632.35500.d9. [DOI] [PubMed] [Google Scholar]
- Di Lisa F, Bernardi P. Mitochondrial function and myocardial aging. A critical analysis of the role of permeability transition. Cardiovasc Res. 2005;66:222–232. doi: 10.1016/j.cardiores.2005.02.009. [DOI] [PubMed] [Google Scholar]
- Di Lisa F, Bernardi P. Mitochondria and ischemia-reperfusion injury of the heart: fixing a hole. Cardiovasc Res. 2006;70:191–199. doi: 10.1016/j.cardiores.2006.01.016. [DOI] [PubMed] [Google Scholar]
- Drew B, Phaneuf S, Dirks A, Selman C, Gredilla R, Lezza A, Barja G, Leeuwenburgh C. Effects of aging and caloric restriction on mitochondrial energy production in gastrocnemius muscle and heart. Am J Physiol Regul Integr Comp Physiol. 2003;284:R474–R480. doi: 10.1152/ajpregu.00455.2002. [DOI] [PubMed] [Google Scholar]
- Ellison GM, Torella D, Karakikes I, Nadal-Ginard B. Myocyte death and renewal: modern concepts of cardiac cellular homeostasis. Nat Clin Pract Cardiovasc Med. 2007;4 1:S52–S59. doi: 10.1038/ncpcardio0773. [DOI] [PubMed] [Google Scholar]
- Fannin SW, Lesnefsky EJ, Slabe TJ, Hassan MO, Hoppel CL. Aging selectively decreases oxidative capacity in rat heart interfibrillar mitochondria. Arch Biochem Biophys. 1999;372:399–407. doi: 10.1006/abbi.1999.1508. [DOI] [PubMed] [Google Scholar]
- Fontana L, Meyer TE, Klein S, Holloszy JO. Long-term calorie restriction is highly effective in reducing the risk for atherosclerosis in humans. Proc Natl Acad Sci U S A. 2004;101:6659–6663. doi: 10.1073/pnas.0308291101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill C, Mestril R, Samali A. Losing heart: the role of apoptosis in heart disease--a novel therapeutic target? FASEB J. 2002;16:135–146. doi: 10.1096/fj.01-0629com. [DOI] [PubMed] [Google Scholar]
- Gredilla R, Lopez-Torres M, Barja G. Effect of time of restriction on the decrease in mitochondrial H2O2 production and oxidative DNA damage in the heart of food-restricted rats. Microsc Res Tech. 2002;59:273–277. doi: 10.1002/jemt.10204. [DOI] [PubMed] [Google Scholar]
- Gredilla R, Sanz A, Lopez-Torres M, Barja G. Caloric restriction decreases mitochondrial free radical generation at complex I and lowers oxidative damage to mitochondrial DNA in the rat heart. FASEB J. 2001;15:1589–1591. doi: 10.1096/fj.00-0764fje. [DOI] [PubMed] [Google Scholar]
- Grimm S, Brdiczka D. The permeability transition pore in cell death. Apoptosis. 2007;12:841–855. doi: 10.1007/s10495-007-0747-3. [DOI] [PubMed] [Google Scholar]
- Hacker TA, McKiernan SH, Douglas PS, Wanagat J, Aiken JM. Age-related changes in cardiac structure and function in Fischer 344 × Brown Norway hybrid rats. Am J Physiol Heart Circ Physiol. 2006;290:H304–H311. doi: 10.1152/ajpheart.00290.2005. [DOI] [PubMed] [Google Scholar]
- Halestrap AP. Calcium, mitochondria and reperfusion injury: a pore way to die. Biochem Soc Trans. 2006;34:232–237. doi: 10.1042/BST20060232. [DOI] [PubMed] [Google Scholar]
- Hansford RG, Hogue BA, Mildaziene V. Dependence of H2O2 formation by rat heart mitochondria on substrate availability and donor age. J Bioenerg Biomembr. 1997;29:89–95. doi: 10.1023/a:1022420007908. [DOI] [PubMed] [Google Scholar]
- Hansford RG, Zorov D. Role of mitochondrial calcium transport in the control of substrate oxidation. Mol Cell Biochem. 1998;184:359–369. [PubMed] [Google Scholar]
- Harman D. The free radical theory of aging. Antioxid Redox Signal. 2003;5:557–561. doi: 10.1089/152308603770310202. [DOI] [PubMed] [Google Scholar]
- Hausenloy DJ, Duchen MR, Yellon DM. Inhibiting mitochondrial permeability transition pore opening at reperfusion protects against ischaemia-reperfusion injury. Cardiovasc Res. 2003;60:617–625. doi: 10.1016/j.cardiores.2003.09.025. [DOI] [PubMed] [Google Scholar]
- Hayflick L. Biological aging is no longer an unsolved problem. Ann N Y Acad Sci. 2007;1100:1–13. doi: 10.1196/annals.1395.001. [DOI] [PubMed] [Google Scholar]
- Hiona A, Leeuwenburgh C. The role of mitochondrial DNA mutations in aging and sarcopenia: Implications for the mitochondrial vicious cycle theory of aging. Exp Gerontol. 2008;43:24–33. doi: 10.1016/j.exger.2007.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichas F, Jouaville LS, Mazat JP. Mitochondria are excitable organelles capable of generating and conveying electrical and calcium signals. Cell. 1997;89:1145–1153. doi: 10.1016/s0092-8674(00)80301-3. [DOI] [PubMed] [Google Scholar]
- Jahangir A, Ozcan C, Holmuhamedov EL, Terzic A. Increased calcium vulnerability of senescent cardiac mitochondria: protective role for a mitochondrial potassium channel opener. Mech Ageing Dev. 2001;122:1073–1086. doi: 10.1016/s0047-6374(01)00242-1. [DOI] [PubMed] [Google Scholar]
- Jekabsone A, Dapkunas Z, Brown GC, Borutaite V. S-nitrosothiol-induced rapid cytochrome c release, caspase activation and mitochondrial permeability transition in perfused heart. Biochem Pharmacol. 2003;66:1513–1519. doi: 10.1016/s0006-2952(03)00506-9. [DOI] [PubMed] [Google Scholar]
- Judge S, Jang YM, Smith A, Hagen T, Leeuwenburgh C. Age-associated increases in oxidative stress and antioxidant enzyme activities in cardiac interfibrillar mitochondria: implications for the mitochondrial theory of aging. FASEB J. 2005a;19:419–421. doi: 10.1096/fj.04-2622fje. [DOI] [PubMed] [Google Scholar]
- Judge S, Jang YM, Smith A, Selman C, Phillips T, Speakman JR, Hagen T, Leeuwenburgh C. Exercise by lifelong voluntary wheel running reduces subsarcolemmal and interfibrillar mitochondrial hydrogen peroxide production in the heart. Am J Physiol Regul Integr Comp Physiol. 2005b;289:R1564–R1572. doi: 10.1152/ajpregu.00396.2005. [DOI] [PubMed] [Google Scholar]
- Judge S, Judge A, Grune T, Leeuwenburgh C. Short-term CR decreases cardiac mitochondrial oxidant production but increases carbonyl content. Am J Physiol Regul Integr Comp Physiol. 2004;286:R254–R259. doi: 10.1152/ajpregu.00502.2003. [DOI] [PubMed] [Google Scholar]
- Judge S, Leeuwenburgh C. Cardiac mitochondrial bioenergetics, oxidative stress, and aging. Am J Physiol Cell Physiol. 2007;292:C1983–C1992. doi: 10.1152/ajpcell.00285.2006. [DOI] [PubMed] [Google Scholar]
- Kajstura J, Cheng W, Sarangarajan R, Li P, Li B, Nitahara JA, Chapnick S, Reiss K, Olivetti G, Anversa P. Necrotic and apoptotic myocyte cell death in the aging heart of Fischer 344 rats. Am J Physiol. 1996;271:H1215–H1228. doi: 10.1152/ajpheart.1996.271.3.H1215. [DOI] [PubMed] [Google Scholar]
- Kroemer G, Galluzzi L, Brenner C. Mitochondrial membrane permeabilization in cell death. Physiol Rev. 2007;87:99–163. doi: 10.1152/physrev.00013.2006. [DOI] [PubMed] [Google Scholar]
- Kujoth GC, Hiona A, Pugh TD, Someya S, Panzer K, Wohlgemuth SE, Hofer T, Seo AY, Sullivan R, Jobling WA, Morrow JD, Van RH, Sedivy JM, Yamasoba T, Tanokura M, Weindruch R, Leeuwenburgh C, Prolla TA. Mitochondrial DNA mutations, oxidative stress, and apoptosis in mammalian aging. Science. 2005;309:481–484. doi: 10.1126/science.1112125. [DOI] [PubMed] [Google Scholar]
- Lesnefsky EJ, Gudz TI, Moghaddas S, Migita CT, Ikeda-Saito M, Turkaly PJ, Hoppel CL. Aging decreases electron transport complex III activity in heart interfibrillar mitochondria by alteration of the cytochrome c binding site. J Mol Cell Cardiol. 2001a;33:37–47. doi: 10.1006/jmcc.2000.1273. [DOI] [PubMed] [Google Scholar]
- Lesnefsky EJ, Moghaddas S, Tandler B, Kerner J, Hoppel CL. Mitochondrial dysfunction in cardiac disease: ischemia--reperfusion, aging, and heart failure. J Mol Cell Cardiol. 2001b;33:1065–1089. doi: 10.1006/jmcc.2001.1378. [DOI] [PubMed] [Google Scholar]
- Leung AW, Halestrap AP. Recent progress in elucidating the molecular mechanism of the mitochondrial permeability transition pore. Biochim Biophys Acta. 2008;1777:946–952. doi: 10.1016/j.bbabio.2008.03.009. [DOI] [PubMed] [Google Scholar]
- Lipman RD, Chrisp CE, Hazzard DG, Bronson RT. Pathologic characterization of brown Norway, brown Norway × Fischer 344, and Fischer 344 × brown Norway rats with relation to age. J Gerontol A Biol Sci Med Sci. 1996;51:B54–B59. doi: 10.1093/gerona/51A.1.B54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manzelmann MS, Harmon HJ. Lack of age-dependent changes in rat heart mitochondria. Mech Ageing Dev. 1987;39:281–288. doi: 10.1016/0047-6374(87)90067-4. [DOI] [PubMed] [Google Scholar]
- McDowell EM, Trump BF. Histologic fixatives suitable for diagnostic light and electron microscopy. Arch Pathol Lab Med. 1976;100:405–414. [PubMed] [Google Scholar]
- Menzies RA, Gold PH. The turnover of mitochondria in a variety of tissues of young adult and aged rats. J Biol Chem. 1971;246:2425–2429. [PubMed] [Google Scholar]
- Meyer TE, Kovacs SJ, Ehsani AA, Klein S, Holloszy JO, Fontana L. Long-term caloric restriction ameliorates the decline in diastolic function in humans. J Am Coll Cardiol. 2006;47:398–402. doi: 10.1016/j.jacc.2005.08.069. [DOI] [PubMed] [Google Scholar]
- Miro O, Casademont J, Casals E, Perea M, Urbano-Marquez A, Rustin P, Cardellach F. Aging is associated with increased lipid peroxidation in human hearts, but not with mitochondrial respiratory chain enzyme defects. Cardiovasc Res. 2000;47:624–631. doi: 10.1016/s0008-6363(00)00122-x. [DOI] [PubMed] [Google Scholar]
- Misare BD, Krukenkamp IB, Levitsky S. Age-dependent sensitivity to unprotected cardiac ischemia: the senescent myocardium. J Thorac Cardiovasc Surg. 1992;103:60–64. [PubMed] [Google Scholar]
- Moghaddas S, Stoll MS, Minkler PE, Salomon RG, Hoppel CL, Lesnefsky EJ. Preservation of cardiolipin content during aging in rat heart interfibrillar mitochondria. J Gerontol A Biol Sci Med Sci. 2002;57:B22–B28. doi: 10.1093/gerona/57.1.b22. [DOI] [PubMed] [Google Scholar]
- Muller-Hocker J. Cytochrome-c-oxidase deficient cardiomyocytes in the human heart--an age-related phenomenon. A histochemical ultracytochemical study. Am J Pathol. 1989;134:1167–1173. [PMC free article] [PubMed] [Google Scholar]
- Muscari C, Caldarera CM, Guarnieri C. Age-dependent production of mitochondrial hydrogen peroxide, lipid peroxides and fluorescent pigments in the rat heart. Basic Res Cardiol. 1990;85:172–178. doi: 10.1007/BF01906970. [DOI] [PubMed] [Google Scholar]
- Nohl H, Hegner D. Do mitochondria produce oxygen radicals in vivo? Eur J Biochem. 1978;82:563–567. doi: 10.1111/j.1432-1033.1978.tb12051.x. [DOI] [PubMed] [Google Scholar]
- Olivetti G, Cigola E, Maestri R, Lagrasta C, Corradi D, Quaini F. Recent advances in cardiac hypertrophy. Cardiovasc Res. 2000;45:68–75. doi: 10.1016/s0008-6363(99)00298-9. [DOI] [PubMed] [Google Scholar]
- Palmer JW, Tandler B, Hoppel CL. Biochemical properties of subsarcolemmal and interfibrillar mitochondria isolated from rat cardiac muscle. J Biol Chem. 1977;252:8731–8739. [PubMed] [Google Scholar]
- Palmer JW, Tandler B, Hoppel CL. Biochemical differences between subsarcolemmal and interfibrillar mitochondria from rat cardiac muscle: effects of procedural manipulations. Arch Biochem Biophys. 1985;236:691–702. doi: 10.1016/0003-9861(85)90675-7. [DOI] [PubMed] [Google Scholar]
- Pepe S. Mitochondrial function in ischaemia and reperfusion of the ageing heart. Clin Exp Pharmacol Physiol. 2000;27:745–750. doi: 10.1046/j.1440-1681.2000.03326.x. [DOI] [PubMed] [Google Scholar]
- Pepe S, Tsuchiya N, Lakatta EG, Hansford RG. PUFA and aging modulate cardiac mitochondrial membrane lipid composition and Ca2+ activation of PDH. Am J Physiol. 1999;276:H149–H158. doi: 10.1152/ajpheart.1999.276.1.H149. [DOI] [PubMed] [Google Scholar]
- Petrosillo G, Ruggiero FM, Pistolese M, Paradies G. Reactive oxygen species generated from the mitochondrial electron transport chain induce cytochrome c dissociation from beef-heart submitochondrial particles via cardiolipin peroxidation. Possible role in the apoptosis. FEBS Lett. 2001;509:435–438. doi: 10.1016/s0014-5793(01)03206-9. [DOI] [PubMed] [Google Scholar]
- Phaneuf S, Leeuwenburgh C. Cytochrome c release from mitochondria in the aging heart: a possible mechanism for apoptosis with age. Am J Physiol Regul Integr Comp Physiol. 2002;282:R423–R430. doi: 10.1152/ajpregu.00296.2001. [DOI] [PubMed] [Google Scholar]
- Pugh KG, Wei JY. Clinical implications of physiological changes in the aging heart. Drugs Aging. 2001;18:263–276. doi: 10.2165/00002512-200118040-00004. [DOI] [PubMed] [Google Scholar]
- Riva A, Tandler B, Loffredo F, Vazquez E, Hoppel C. Structural differences in two biochemically defined populations of cardiac mitochondria. Am J Physiol Heart Circ Physiol. 2005;289:H868–H872. doi: 10.1152/ajpheart.00866.2004. [DOI] [PubMed] [Google Scholar]
- Sohal RS, Ku HH, Agarwal S, Forster MJ, Lal H. Oxidative damage, mitochondrial oxidant generation and antioxidant defenses during aging and in response to food restriction in the mouse. Mech Ageing Dev. 1994;74:121–133. doi: 10.1016/0047-6374(94)90104-x. [DOI] [PubMed] [Google Scholar]
- Sprott RL, Austad SN. Animal Models for Aging Research. In: Schneider EL, Rowe JW, editors. Handbook of the Biology of Aging. 4th. San Diego: Academic Press Inc.; 1996. pp. 3–23. [Google Scholar]
- Suh JH, Heath SH, Hagen TM. Two subpopulations of mitochondria in the aging rat heart display heterogenous levels of oxidative stress. Free Radic Biol Med. 2003;35:1064–1072. doi: 10.1016/s0891-5849(03)00468-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taffet GE, Pham TT, Hartley CJ. The age-associated alterations in late diastolic function in mice are improved by caloric restriction. J Gerontol A Biol Sci Med Sci. 1997;52:B285–B290. doi: 10.1093/gerona/52a.6.b285. [DOI] [PubMed] [Google Scholar]
- Tanveer A, Virji S, Andreeva L, Totty NF, Hsuan JJ, Ward JM, Crompton M. Involvement of cyclophilin D in the activation of a mitochondrial pore by Ca2+ and oxidant stress. Eur J Biochem. 1996;238:166–172. doi: 10.1111/j.1432-1033.1996.0166q.x. [DOI] [PubMed] [Google Scholar]
- Wohlgemuth SE, Julian D, Akin DE, Fried J, Toscano K, Leeuwenburgh C, Dunn WA. Autophagy in the heart and liver during normal aging and calorie restriction. Rejuvenation Res. 2007;10:281–292. doi: 10.1089/rej.2006.0535. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.