Fig. 2.
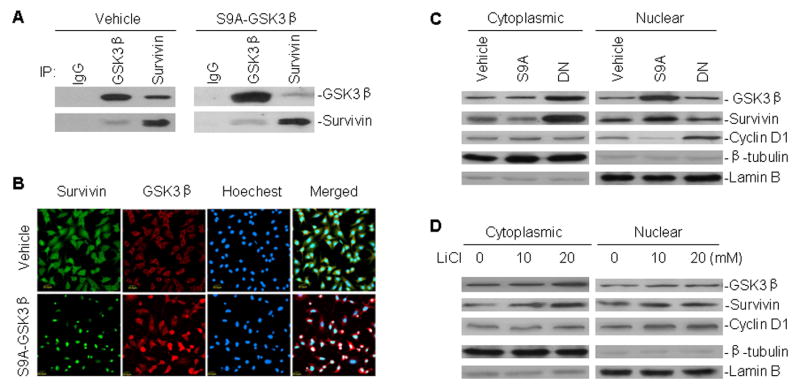
Interaction between GSK3β and survivin, and their subcellular distribution in A549 cells transfected with GSK3β mutant plasmids and treated with LiCl. A, binding of GSK3β to survivin in A549 cells. Cell extracts from A549 transfected with either S9A-GSK3β or control vehicle were immunoprecipitated with antibodies as indicated. Normal mouse or rabbit antibodies served as control (IgG). Proteins in immune complexes were separated on denaturing gels, transferred to filters, and detected by western blotting with anti-GSK3β and anti-survivin antibodies. Western blot and IP antibodies were from different species. B, A549 cells transfected with S9A-GSK3β and vehicle were grown on coverslips for 24 h, fixed in absolute methanol, processed by immunofluorescence staining, and detected by confocal microscopy for the interaction between GSK3β and survivin. C, cytoplasmic and nuclear extracts of A549 cells transfected with S9A-GSK3β (S9A) and DN-GSK3β (DN), subjected to western blot analysis for GSK3β, survivin and cyclin D1 expression. Lamin B and β-tubulin served as controls for equal protein loading of nuclear and cytoplasmic fractions, respectively. D, Western blot assay similar to (C), for subcellular distribution of GSK3β, survivin and cyclin D1 in A549 cells treated with different concentrations of LiCl, as indicated, for 24 h.
