Abstract
AIM: To investigate the feasibility of detecting hypermethylated secreted frizzled-related protein 2 (SFRP2) gene in fecal DNA as a non-invasive screening tool for colorectal cancer (CRC).
METHODS: Fluorescence-based real-time PCR assay (MethyLight) was performed to analyze SFRP2 gene promoter methylation status in a blinded fashion in tumor tissues and in stool samples taken from 69 CRC patients preoperatively and at the 9th postoperative day, 34 patients with adenoma ≥ 1 cm, 26 with hyperplastic polyp, and 30 endoscopically normal subjects. Simultaneously the relationship between hypermethylation of SFRP2 gene and clinicopathological features was analyzed.
RESULTS: SFRP2 gene was hypermethylated in 91.3% (63/69) CRC, 79.4% (27/34) and 53.8% (14/26) adenoma and hyperplastic polyp tissues, and in 87.0% (60/69), 61.8% (21/34) and 42.3% (11/26) of corresponding fecal samples, respectively. In contrast, no methylated SFRP2 gene was detected in mucosal tissues of normal controls, while two cases of matched fecal samples from normal controls were detected with hypermethylated SFRP2. A significant decrease (P < 0.001) in the rate of hypermethylated SFRP2 gene was detected in the postoperative (8.7%, 6/69) fecal samples as compared with the preoperative fecal samples (87%, 60/69) of CRC patients. Moreover, no significant associations were observed between SFRP2 hypermethylation and clinicopathological features including sex, age, tumor stage, site, lymph node status and histological grade, etc.
CONCLUSION: Hypermethylation of SFRP2 gene in fecal DNA is a novel molecular biomarker of CRC and carries a high potential for the remote detection of CRC and premalignant lesions as noninvasive screening method.
Keywords: Colorectal cancer, Secreted frizzled-related protein 2, Feces, Methylation, Screening
INTRODUCTION
Colorectal cancer (CRC) is the third most common cancer worldwide, and is the second major cause of death from cancer in Europe and in the USA[1,2]. Worldwide almost one million new cases occur annually, amounting to 492 000 related deaths[3]. However, the incidence and mortality of CRC have been decreasing over the last decade, and this may be attributable to more effective screening and surveillance[2]. CRC is most effectively treated when diagnosed at an early stage, but the early stage of CRC is mostly symptomless as well as delitescence nature. Approximately 40% of colorectal cancers are diagnosed with localized disease, which have approximately a 90% five-year survival rate after curative surgery. However, the prognosis worsens with advancing stage, and only 5% of patients diagnosed with distant metastases survive 5 years[4]. With the potential of both reducing mortality from CRC as well as enhancing primary prevention through detection and removal of lesions that could potentially develop into cancer, early detection of CRC will increase survival the most[5]. Therefore, the need for early detection is clear, and an effective screening test would have substantial clinical benefits.
A promising noninvasive screening tool for colorectal neoplasia detection is to assay for molecular biomarkers that represent a “specific” or “spectrum” of genetic and/or epigenetic alterations existing in gastrointestinal tumor cells shed into stool[6]. Potentially, both premalignant adenomas and CRC may be detected this way. Epigenetic alterations are defined as heritable signatures of information other than nucleotide sequences. The interest in this field (especially DNA methylation) is immense, and the past several years have seen an explosion in investigations based on the unique differences in methylation patterns between the cancerous and normal-appearing tissues, and this new assay facilitates its use for noninvasive CRC screening[7,8]. However, in contrast to the relatively well accepted mutation-based fecal DNA testing[9], methylation-based testing has been initiated more recently, and is beginning to identify adequate specific markers that are representative of epigenetic “signature” alterations. Methylation analysis of a number of gene promoters in DNA from stool samples has been less comprehensively investigated, but has been suggested to be a sensitive diagnostic tool for colorectal tumor[10–14].
Aberrant Wnt pathway signaling is an early progression event in up to 90% of CRCs[15]. Suzuki et al[16–18] have identified frequent promoter hypermethylation and gene silencing of the secreted frizzled-related protein (SFRP) genes in CRC, in which SFRP2 gene at human chromosome 4q31.3 is claimed as a tumor suppressor gene inactivated by the epigenetic CpG hypermethylation in CRC[19]. SFRP2 is split in three exons of variable size separated by two introns, and have dense CpG islands around its first exons[16]. The CpG islands were closed correlated with DNA methylation of tumor cells. Its expression reportedly is inhibited in CRC specimens by hypermethylation of a CpG island extending from the gene’s 5’-flanking region through the first exon[18]. In CRC, SFRP2 genes have been identified that are commonly unmethylated and expressed in normal colon mucosa but are methylated and silenced in CRC[16–18,20]. Detection of hypermethylation of SFRP2 genes in human DNA isolated from stools might provide a novel strategy for the detection and investigation of CRC.
In the present study, we analyzed methylation of SFRP2 in stools taken from CRC patients preoperatively and postoperatively, and from patients with benign colorectal diseases as well as from normal controls, and then evaluated the potential of fecal SFRP2 gene hypermethylation as a non-invasive screening tool for CRC.
MATERIALS AND METHODS
Patients and collection of tissue and fecal DNA samples
Colorectal tissue samples were routinely collected from 69 patients with sporadic CRC, 60 patients with benign colorectal diseases (34 adenomas and 26 hyperplastic polyps) and 30 macroscopically normal subjects undergoing surgery and endoscopy at the First Affiliated Hospital of Yangzhou university from March 2005 to February 2007. Meanwhile, the matched fecal samples were collected from all CRC patients preoperatively at the time bowel preparation and at the 9th postoperative day, from the patients with benign colorectal diseases and from the endoscopically normal patients, and were kept at 4°C until being processed. Within 12 h after collection, the samples were washed with 1 × PBS and centrifuged at 1800 r/min for 15 min to pellet the solid stool. The pellet of stool and tissue samples were all stored at -80°C. None of the patients had received chemotherapy or radiation therapy prior to surgery. All patients gave informed consent for their participation in the study which had been approved by the Ethical Committee of our university.
DNA isolation from tissue and fecal samples
Samples were randomly coded before processing to ensure adequate blinding of the clinical information. DNA was isolated from colonic tissues (5-10 mg) by using QIAamp DNA Mini Kit (Qiagen) and from stool samples (250 mg) by using QIAamp DNA Stool Mini Kit (Qiagen) according to the manufacturer’s protocol. HE-stained sections from tissue samples were examined by an experienced pathologist to confirm the histological diagnosis.
Bisulfite modification
The DNA was chemically modified by sodium bisulphate to convert all unmethylated cytosines to uracils while leaving methylcytosines unaltered (EpiTect® Bisulfite Kit, Qiagen), and eluted in 50 μL of elution buffer.
Methylation analysis
The bisulfite-modified DNA was used as a template for fluorescence-based real-time PCR (MethyLight) as described previously[21,22]. SFRP2 gene was examined, and template-free distilled water was included as a negative control for amplification. The sequences of primers reported previously are listed in Table 1[14,23].
Table 1.
SFRP2 gene primer sequences, annealing temperature and product size for MethyLight assays
| CpG status | Forward primer (5’-3’) | Reverse primer (5’-3’) | Annealing temperature (°C) | Product size (bp) |
| M | GGGTCGGAGTTTTTCGGAGTTGCGC | CCGCTCTCTTCGCTAAATACGACTCG | 62 | 138 |
| U | TTTTGGGTTGGAGTTTTTTGGAGTTGTGT | AACCCACTCTCTTCACTAAATACAACTCA | 50 | 145 |
M: Methylated; U: Unmethylated.
For the MethyLight, 3 μL of bisulfite-converted DNA was used in each amplification. PCR was performed in a reaction volume of 25 μL consisting of 17.875 μL of ddH2O, 2.5 μL of 10 × PCR buffer, 2 μL of dNTP mixture, 0.25 μL of forward primer, 0.25 μL of reverse primer, 2 μL of template, and 0.125 μL of TaKaRa Taq HS (Hot Start) at the following conditions: pre-denaturation at 95°C for 5 min, followed by 40 amplification cycles of denaturation at 95°C for 30 s, primer annealing at 50°C for 30 s (U primer), and extension at 62°C for 30 s (M primer), and finally a further extension at 72°C for 5 min. The stain was SYBR greenIand PCR was performed in the LightCycle Fluorescent Quantitation PCR Detection System (LightCycler 1.0, Switzerland). A standard curve was created by plotting the logarithmic amount of the standard universal methylated DNA (31.25 pg to 10 ng) against the threshold cycle (CT) value.
Statistical analysis
To compare characteristics of the different groups of patients and biologic samples, two sided Student’s t test, Chi-square test, and Fisher exact test were used as appropriate. Data were analyzed using SPSS10.0 software. P < 0.05 was considered statistically significant.
RESULTS
Hypermethylation of SFRP2 gene in hyperplastic polyps, advanced adenomas and colorectal cancers
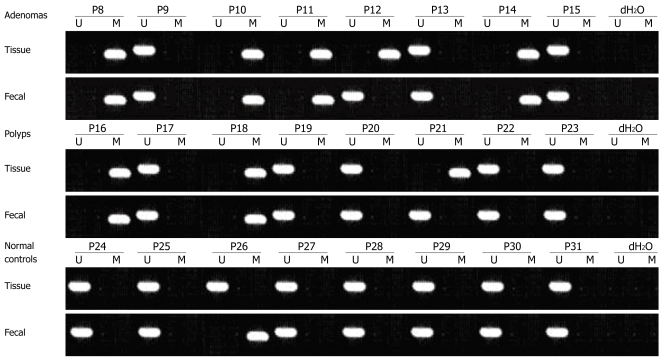
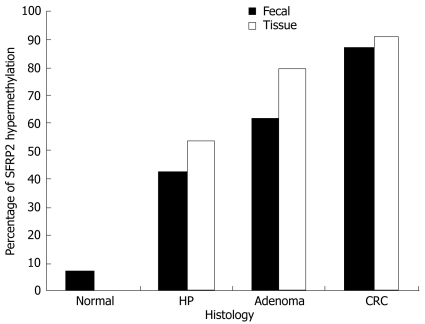
We examined hypermethylation of SFRP2 gene in DNA from 69 patients with CRC, 34 with advanced adenoma (characterized by size ≥ 1 cm, villous histology, and high-grade dysplasia) and 26 with hyperplastic polyps. SFRP2 was hypermethylated in 91.3% (63/69), 79.4% (27/34) and 53.8% (14/26) of the CRCs, advanced adenomas and hyperplastic polyps, respectively. In the colorectal advanced adenomas, the prevalence of hypermethylated SFRP2 was substantially higher in the tubulovillous and villous adenomas as compared with the tubular adenomas, and was much more in the adenomas accompanying with epithelium dysplasia. The prevalence of hypermethylated SFRP2 had a gradually increased tendency from hyper-plastic polyps to CRCs. In contrast, none of the normal colorectal mucosa of endoscopically normal patients showed any methylated bands (Figures 1, 2 and 3, Table 2).
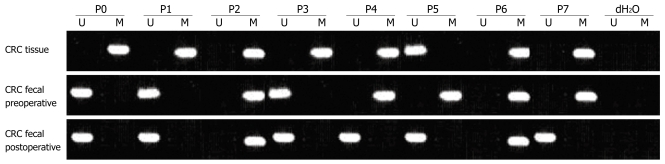
Figure 1.
Methylation status of SFRP2 gene in preoperative and postoperative fecal specimens and in CRC tissues taken from the same patients. P: Patient; M: Methylated; U: Unmethylated.
Figure 2.
Methylation status of SFRP2 gene in tissue and feces taken from the same patients with benign colorectal diseases and normal controls.
Figure 3.
Prevalence of hypermethylated SFRP2 in stool and tissue specimens taken from the subjects with normal colonic mucosa, hyperplastic polyps (HP), adenomas and colorectal cancers (CRCs).
Table 2.
Hypermethylation of SFRP2 in tissue and stool specimens taken from the same patients with colorectal cancer, adenoma, polyp and normal controls
| Characteristics | Case (n) | Tissue M1 (%) | χ2 | P | Fecal M2 (%) | χ2 | P | Sensitivity M2/n | Specificity |
| Colorectal Cancer | 69 | 63 (91.3) | 75.3261 | 0.0001 | 60 (87.0) | 57.5881 | 0.0001 | 87.00% | |
| Age | |||||||||
| < 50 | 20 | 17 (85.0) | 1.410 | 0.235 | 16 (80.0) | 1.202 | 0.273 | ||
| ≥ 50 | 49 | 46 (93.9) | 44 (89.8) | ||||||
| Sex | |||||||||
| Male | 37 | 34 (91.9) | 0.035 | 0.852 | 32 (86.5) | 0.016 | 0.901 | ||
| Female | 32 | 29 (90.6) | 28 (87.5) | ||||||
| TNM stage | |||||||||
| I/II | 30 | 27 (90.0) | 0.114 | 0.736 | 25 (83.3) | 0.614 | 0.433 | ||
| III/IV | 39 | 36 (92.3) | 35 (89.7) | ||||||
| Lymph node status | |||||||||
| Positive | 27 | 25 (92.6) | 0.093 | 0.761 | 24 (88.9) | 0.146 | 0.702 | ||
| Negative | 42 | 38 (90.5) | 36 (85.7) | ||||||
| Infiltration | |||||||||
| Mucosa underlayer | 21 | 18 (85.7) | 1.188 | 0.276 | 17 (81.0) | 0.959 | 0.327 | ||
| Muscular coat | 48 | 45 (93.8) | 43 (89.6) | ||||||
| Tumor site | |||||||||
| Rectum | 30 | 27 (90.0) | 0.306 | 0.858 | 27 (90.0) | 0.428 | 0.786 | ||
| Left hemicolon | 18 | 17 (94.4) | 15 (83.3) | ||||||
| Right hemicolon | 21 | 19 (90.5) | 18 (85.7) | ||||||
| Adenoma | 34 | 27 (79.4) | 27.5791 | 0.0001 | 21 (61.8) | 21.0161 | 0.0001 | 61.80% | |
| Tubular adenoma | 11 | 8 (72.7) | 0.518 | 0.772 | 6 (54.5) | 0.563 | 0.755 | ||
| Villous adenoma | 10 | 8 (80.0) | 6 (60.0) | ||||||
| Tubulovillous adenoma | 13 | 11 (84.6) | 9 (69.2) | ||||||
| Hyperplastic polyp | 26 | 14 (53.8) | 21.5381 | 0.0001 | 11 (42.3) | 9.9261 | 0.0021 | 42.30% | 76.80% |
| Normal control | 30 | 0 | 2 (6.7) |
M1: Hypermethylated SFRP2 in tissue; M2: Hypermethylated SFRP2 in stool.
Represents the value of χ2 and P vs normal controls. There were significant differences in prevalence of hypermethylated SFRP2 in tissue and stool between cancer and adenoma (χ2 = 2.921, P = 0.087 and χ2 = 8.606, P = 0.003, respectively) and between cancer and hyperplastic polyp (χ2 = 17.253, P < 0.001 and χ2 = 19.936, P < 0.001, respectively). However, a significant difference was observed in prevalence of hypermethylated SFRP2 between adenoma and hyperplastic polyp only in tissue (χ2 = 4.450, P = 0.035) but not in fecal specimens (χ2 = 2.241, P = 0.134).
The hypermethylation of SFRP2 gene was significantly different between cancer and normal mucosa (P < 0.001), between advanced adenoma and normal mucosa (P < 0.001), between hyperplastic polyp and normal mucosa (P < 0.001), between cancer and hyperplastic polyp (P < 0.001), and between adenoma and hyperplastic polyp (P = 0.035); however, no significant difference was found between cancer and adenoma (P = 0.087) (Table 2).
Detection of SFRP2 hypermethylation in fecal DNA
Following the performance of the MethyLight assays on the DNA extracted from the tissue samples, we next assessed matched fecal DNA from the corresponding patients to detect SFRP2 hypermethylation in fecal DNA from individuals with CRC or premalignant lesions. We found that 92.1% (58/63) of CRCs, 66.7% (18/27) of advanced adenomas and 71.4% (10/14) of hyperplastic polyps had concordant hypermethylated SFRP2 in their tissue DNA and fecal DNA, respectively. Two patients with CRC, 3 with advanced adenoma and 1 with hyperplastic polyp had methylated SFRP2 only in the fecal DNA, while 5 patients with CRC, 9 with advanced adenoma and 4 with hyperplastic polyp had methylated SFRP2 only in the tissue DNA. All of the remaining subjects that had unmethylated SFRP2 in the tissue DNA showed unmethylated SFRP2 in their fecal samples (Figures 1, 2, 3 and 4, Table 2).
Figure 4.
The photofluorogram of hypermethylated SFRP2 gene in fecal specimen performed by MethyLight. A: Photofluorogram of SFRP2 gene performed in M (methylation) primer and its amplification condition; B: Photofluorogram of SFRP2 gene performed in U (unmethylation) primer and its amplification condition. P represents the patients; and NTC represents the negative controls.
Comparison of the performance characteristics of SFRP2 MethyLight assays showed that the assays could detect 92.1%, 66.7% and 71.4% of individuals with CRC, advanced adenoma and hyperplastic polyp, respectively, that carried hypermethylated SFRP2. The clinical sensitivities of SFRP2 hypermethylation in fecal DNA for detecting the presence of CRC, advanced adenoma and hyperplastic polyp were 87.0% (60/69), 61.8% (21/34) and 42.3% (11/26), respectively. To evaluate the clinical specificity of this assay, we next analyzed fecal DNA of 30 normal control individuals, and found that only 2 (6.7%) samples were positive for hypermethylation of SFRP2 (Table 2).
Detection of SFRP2 hypermethylation in fecal DNA of CRC patients preoperatively and postoperatively
We compared the SFRP2 hypermethylation in fecal DNA collected preoperatively and at the 9th postoperative day from CRC patients. Five of the 60 CRC patients whose stool carried hypermethylated SFRP2 preoperatively had detectable hypermethylated SFRP2 in their fecal samples postoperatively, showing a significant decrease postoperatively (P < 0.001). Only one of the nine CRC patients whose stool carried unmethylated SFRP2 preoperatively had detectable hypermethylated SFRP2 in the fecal sample postoperatively. All of the remaining 8 CRC patients with fecal unmethylated SFRP2 preoperatively showed fecal unmethylated SFRP2 postoperatively.
Moreover, no significant associations were obser-ved between SFRP2 gene hypermethylation and clinicopathological features, including sex, age, tumor stage, site, lymph node status and histological grade, etc (Table 2).
DISCUSSION
CRC is a common malignancy that arises from benign neoplasms and evolves into adenocarcinomas through a stepwise histological progression sequence, proceeding from either adenomas or hyperplastic polyps/serrated adenomas[24–26]. Genetic alterations have been associated with specific steps in this adenoma-carcinoma sequence and are believed to drive the histological progression of CRC[27]. Recently, epigenetic alterations, especially DNA methylation, have been shown to occur in colorectal polyps, adenomas and CRCs[28–30]. The aberrant methylation of genes appears to act together with genetic alterations to drive the initiation and progression of colorectal polyp and adenoma to CRC[31]. Genetic alterations occur during the adenoma-carcinoma sequence of colorectal cancer formation and drive the initiation and progression of colorectal cancer formation. The aberrant methylation of genes is an alternate, epigenetic mechanism for silencing tumor suppressor genes in colorectal cancer. Thus, the aberrant methylation of genes appears to increase most significantly during the progression of early adenomas to advanced adenomas, and the frequency of specific gene methylation at the different steps of the adenoma-carcinoma progression sequence varies in a gene-specific fashion[28]. DNA methylation changes have been recognized as a key mechanism of colorectal carcinogenesis. Because of the ubiquity of DNA methylation changes and the ability to detect methylated DNA in stool, analysis of aberrantly methylation in stool DNA might provide a novel strategy for noninvasive detection of CRC[7,32–34]. Due to the heterogeneity of tumors, usually multiple markers distributed throughout the human genome need to be analyzed, and this is labor-intensive and does not allow for high throughput screening. Therefore, markers with high sensitivity and good specificity are needed[34]. SFRP2 is one of the soluble modulators of a putative tumorigenic pathway-the Wnt signaling pathway, and is frequently hypermethylated in colorectal cancer, adenoma and aberrant crypt foci (ACF)[16–18,20,35]. At present, there are only a few researches in literature regarding SFRP2 hypermethylation in CRC tissue and in stool of CRC patients[14,20,33]. In this study, we assessed the methylation status of SFRP2 in tissue and matched stool samples from patients with CRC with respect to a series of healthy individuals and patients with benign colorectal diseases, using fluorescence-based real-time PCR assay (MethyLight). Our results suggested SFRP2 hypermethylation as a highly sensitive and specific independent marker for the fecal screening of CRC.
It has been shown that hypermethylation of SFRP2 genes, inhibitors of Wnt signaling pathway[16–18,20], is a common early event in the evolution of colorectal tumor, occurring frequently in ACF, hyperplastic polyp and adenoma, which is regarded as the earliest lesion of multistage colorectal carcinogenesis. It appears that hypermethylation of SFRP2 gene might serve as indicator for early CRC[10,14,33]. We detected hypermethylation of SFRP2 in tissue and matched fecal samples from the corresponding patients with CRC and premalignant diseases. The results showed that SFRP2 was hypermethylated in 91.3%, 79.4% and 53.8% of the CRCs, advanced adenomas and hyperplastic polyps, respectively, thereby indicating that hypermethylation of SFRP2 occurs in hyperplastic polyps and becomes more frequent in advanced adenomas and then in colorectal cancers. Moreover, the clinical sensitivities for detecting the presence of CRC, advanced adenoma and hyperplastic polyp were 87.0%, 61.8% and 42.3%, respectively, and the overall specificity was 76.8%. In addition, there was a trend, although not statistically significant, of SFRP2 hypermethylation more among the adenomas accompany with epithelium dysplasia. On contrary, hypermethylation of SFRP2 gene was found in stool of two endoscopically normal subjects, which might be most likely owing to the SFRP2 methylation occurring frequently in premalignant aberrant crypt foci (ACF) that is overlooked at colonoscopy[14,16–18]. Polyp and adenoma are the early events of colorectal tumorigenesis, and have a high risk of malignant transformation[28,36]. Our results show that hypermethylated SFRP was detectable not only in stools of patients with CRC, but also in stools of patients with premalignant lesions, such as advanced adenomas, suggesting that hypermethylated SFRP is a sensitive fecal-based molecular marker for screening CRC and premalignant lesions.
At present, there are only two institutions which had studied the methylated SFRP2 in stool as a molecule marker for CRC screening: Müller et al[10] proposed SFRP2 hypermethylation as a sensitive marker which is able to detect 77%-90% of CRCs; and Huang et al[14,33] reported that methylated SFRP2 occurred in 94.2%, 52.4%, 37.5%, and 16.7% of patients with CRC, adenoma, hyperplastic polyp, and ulcerative colitis, respectively. In this study, we simultaneously detected hypermethylated SFRP2 in tissue and fecal samples taken from the patients with CRC and benign colorectal diseases and from the healthy individuals. When compared with the two aforementioned researches, our results showed that hypermethylated SFRP2 in stool had almost the same high sensitivity for detecting CRC, but had significantly higher detection rate for hyperplastic polyp and advanced adenoma. With the same ethnic groups, the discrepancy in performance of our study and that of Huang et al[14,33] is likely attributable to the more matched fecal and tissue samples of the same patients, but the use of a more sensitive methylation assay, MethyLight[21,37–39], may also account for the higher sensitivity and lower specificity. Despite of that, the high level of SFRP2 hypermethylation in patients with hyperplastic polyps is somewhat surprising. There is little evidence that the hyperplastic polyps, which are usually not thought to be premalignant, with methylated DNA would develop into CRC; therefore the hyperplastic polyps will be counted as false positive when using SFRP2 hypermethylation-based screening strategy. Whether hypermethylation of SFRP2 gene can really identify this non-malignant diseases is still unclear. As DNA methylation is more common in advanced adenomas and CRC[40], all our findings and that of Huang et al[14,33] collectively suggest that SFRP2 hypermethylation may reflect the malignant potential of these lesions. Because of the nature of CRC evolution (hyperplastic polyp -serrated adenoma-carcinoma sequence), the levels of hypermethylated SFRP2 in stool of patients with hyperplastic polyps may have substantial clinical benefits through the detection and endoscopic removal of this benign disease, which needs large sample to further study. In short, patients with fecal hypermethylated SFRP2 may belong to high-risk individuals and need more accurate clinical examinations and follow-up.
Furthermore, we compared the hypermethylated SFRP2 gene in stool of CRC patients preoperatively and at the 9th postoperative day. The results indicated that the prevalence of hypermethylated SFRP2 in stool of CRC patients decreased significantly postoperatively, which showed a new evidence that the hypermethylated SFRP2 gene in stool comes from the CRC tissues and SFRP2 hypermethylation in stool carries a high potential for the remote detection of CRC as a noninvasive screening method.
At present, there are several methods for detecting of CRC and premalignant lesions[41,42], but none of them is really suitable for screening CRC. Fecal occult blood testing has been shown to reduce CRC-related mortality and is cheap but has low sensitivity. Colonoscopy, with a high sensitivity and specificity for detection of CRC and large adenomas, requires a thorough bowel preparation, causes discomfort and small but non-negligible risk of major complications in patients, and is an invasive procedure. CT colonography[43], also known as virtual colonoscopy, looks promising but so far has been performed unevenly in the hands of community radiologists. However, aberrant methylation in fecal DNA as a molecular marker has developed a new way for screening CRC and premalignant lesions. Our study demonstrated initially that hypermethylated SFRP2 gene in stool is a promising and noninvasive sensitive marker for screening colorectal neoplasia.
COMMENTS
Background
Silence of secreted frizzled-related protein 2 (SFRPs) genes, which are secreted glycoproteins working as inhibitory modulators of the Wnt signaling pathway induced by promoter hypermethylation, plays a key role in colorectal tumorigenesis, and hypermethylation of SFRPs genes is a common early event in the evolution of colorectal cancer (CRC). SFRP2 is one of the SFRPs gene family members and is frequently hypermethylated in ACF, adenoma and colorectal cancer. Thus, detection of hypermethylation of SFRPs genes in human DNA isolated from stools might provide a novel strategy for the detection and investigation of CRC.
Research frontiers
Epigenetic hypermethylation can result in transcriptional silencing of tumor suppressor genes and is considered to be a key event of sporadic CRC, and gradually become the important areas in the research of CRC. Detection of hypermethylation of SFRPs genes in human DNA isolated from stools might provide a novel strategy for the detection and investigation of CRC.
Innovations and breakthroughs
A more sensitive methylation assay (MethyLight) was performed to analyze SFRP2 gene promoter methylation status in a blinded fashion in tumor tissues and in matched stool samples from the same patients with CRC and colorectal benign diseases. Furthermore, we compared the hypermethylated SFRP2 gene in stool of patients with CRC preoperatively and at the 9th postoperative day.
Applications
Our study demonstrated initially that hypermethylated SFRP2 gene in stool is a promising and noninvasive sensitive single marker for screening colorectal neoplasia.
Terminology
Wnt signaling pathway: Signals are transduced by Wnt ligands through frizzled (Fz) membrane receptors by competition with Fzs for Wnt ligands or by direct formation of nonsignaling complexes with Fzs themselves. Secreted frizzled-related proteins (SFRPs): These are secreted proteins of about 36 ku that receive their name by their structural homology with the extracellular cystein-rich domain (CRD) of frizzled (Fz), a family of developmentally important signaling molecules, and have the ability to bind to both Wnt and Fz proteins. Epigenetic mechanism: Somatic cells acquire changes in gene expression that are transmissible through mitosis but which do not involve any alterations to the DNA sequence. CpG island DNA methylation: The aberrant DNA methylation affects CpG-rich regions, called ‘‘CpG islands’’, in the 5’ region of genes and results in transcriptional silencing through complex effects on transcription factor binding and associated changes in chromatin structure.
Peer review
This paper studied the use of hypermethylation of SFRP2 promoter as a means for diagnosing colorectal cancer. The authors showed that, compared to individuals with normal colon, patients with colon cancer, advanced adenoma and hyperplastic polyps had a high prevalence of SFRP2 hypermethylation in the tissues and stools. There also appeared to be a significant difference in the methylation patterns in patients with colon cancer before and after surgery. They conclude that hypermethylation of SFRP2 may be a sensitive diagnostic test for colorectal neoplasm.
Acknowledgments
We thank Hai-Hang Zhu, Wei Lu and Xiao-Ling Wang for technical support and the volunteers for cooperation.
Supported by The Grant from Programs of Science and Technology Commission Foundation of Jiangsu Province, No. BS2005036
Peer reviewers: Javier S Martin, Gastroenterology and Endoscopy, Sanatorio Cantegril, Av. Roosevelt y P 13, Punta del Este 20100, Uruguay; Vincent W Yang, Professor and Director, 201 Whitehead Research Building, 615 Michael Street, Atlanta, GA 30322, United States; Marc Peeters, MD, PhD, Professor, Department of HepatoGastroenterology, Digestive Oncology Unit, University Hospital Ghent, De Pintelaan 185, B-9000 Gent, Belgium
S- Editor Zhu LH L- Editor Kumar M E- Editor Li HY
References
- 1.Jemal A, Siegel R, Ward E, Murray T, Xu J, Thun MJ. Cancer statistics, 2007. CA Cancer J Clin. 2007;57:43–66. doi: 10.3322/canjclin.57.1.43. [DOI] [PubMed] [Google Scholar]
- 2.Ferlay J, Autier P, Boniol M, Heanue M, Colombet M, Boyle P. Estimates of the cancer incidence and mortality in Europe in 2006. Ann Oncol. 2007;18:581–592. doi: 10.1093/annonc/mdl498. [DOI] [PubMed] [Google Scholar]
- 3.Weitz J, Koch M, Debus J, Hohler T, Galle PR, Buchler MW. Colorectal cancer. Lancet. 2005;365:153–165. doi: 10.1016/S0140-6736(05)17706-X. [DOI] [PubMed] [Google Scholar]
- 4.Pfister DG, Benson AB 3rd, Somerfield MR. Clinical practice. Surveillance strategies after curative treatment of colorectal cancer. N Engl J Med. 2004;350:2375–2382. doi: 10.1056/NEJMcp010529. [DOI] [PubMed] [Google Scholar]
- 5.Rennert G. Prevention and early detection of colorectal cancer--new horizons. Recent Results Cancer Res. 2007;174:179–187. doi: 10.1007/978-3-540-37696-5_15. [DOI] [PubMed] [Google Scholar]
- 6.Nagasaka T, Goel A, Matsubara N, Tanaka N. Detection of fecal DNA methylation for colorectal neoplasia: Does it lead to an optimal screening test? Acta Med Okayama. 2006;60:249–256. doi: 10.18926/AMO/30741. [DOI] [PubMed] [Google Scholar]
- 7.Itzkowitz SH, Jandorf L, Brand R, Rabeneck L, Schroy PC 3rd, Sontag S, Johnson D, Skoletsky J, Durkee K, Markowitz S, et al. Improved fecal DNA test for colorectal cancer screening. Clin Gastroenterol Hepatol. 2007;5:111–117. doi: 10.1016/j.cgh.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 8.von Delius S, Jung A, Schmid RM. Fecal methylated DNA for the detection of colorectal cancer. Z Gastroenterol. 2006;44:1001–1002. doi: 10.1055/s-2006-927124. [DOI] [PubMed] [Google Scholar]
- 9.Imperiale TF, Ransohoff DF, Itzkowitz SH, Turnbull BA, Ross ME; Colorectal Cancer Study Group. Fecal DNA versus fecal occult blood for colorectal-cancer screening in an average-risk population. N Engl J Med. 2004;351:2704–2714. doi: 10.1056/NEJMoa033403. [DOI] [PubMed] [Google Scholar]
- 10.Muller HM, Oberwalder M, Fiegl H, Morandell M, Goebel G, Zitt M, Muhlthaler M, Ofner D, Margreiter R, Widschwendter M. Methylation changes in faecal DNA: a marker for colorectal cancer screening? Lancet. 2004;363:1283–1285. doi: 10.1016/S0140-6736(04)16002-9. [DOI] [PubMed] [Google Scholar]
- 11.Chen WD, Han ZJ, Skoletsky J, Olson J, Sah J, Myeroff L, Platzer P, Lu S, Dawson D, Willis J, et al. Detection in fecal DNA of colon cancer-specific methylation of the nonexpressed vimentin gene. J Natl Cancer Inst. 2005;97:1124–1132. doi: 10.1093/jnci/dji204. [DOI] [PubMed] [Google Scholar]
- 12.Brenner DE, Rennert G. Fecal DNA biomarkers for the detection of colorectal neoplasia: attractive, but is it feasible? J Natl Cancer Inst. 2005;97:1107–1109. doi: 10.1093/jnci/dji244. [DOI] [PubMed] [Google Scholar]
- 13.Petko Z, Ghiassi M, Shuber A, Gorham J, Smalley W, Washington MK, Schultenover S, Gautam S, Markowitz SD, Grady WM. Aberrantly methylated CDKN2A, MGMT, and MLH1 in colon polyps and in fecal DNA from patients with colorectal polyps. Clin Cancer Res. 2005;11:1203–1209. [PubMed] [Google Scholar]
- 14.Huang ZH, Li LH, Yang F, Wang JF. Detection of aberrant methylation in fecal DNA as a molecular screening tool for colorectal cancer and precancerous lesions. World J Gastroenterol. 2007;13:950–954. doi: 10.3748/wjg.v13.i6.950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fodde R, Smits R, Clevers H. APC, signal transduction and genetic instability in colorectal cancer. Nat Rev Cancer. 2001;1:55–67. doi: 10.1038/35094067. [DOI] [PubMed] [Google Scholar]
- 16.Suzuki H, Gabrielson E, Chen W, Anbazhagan R, van Engeland M, Weijenberg MP, Herman JG, Baylin SB. A genomic screen for genes upregulated by demethylation and histone deacetylase inhibition in human colorectal cancer. Nat Genet. 2002;31:141–149. doi: 10.1038/ng892. [DOI] [PubMed] [Google Scholar]
- 17.Suzuki H, Watkins DN, Jair KW, Schuebel KE, Markowitz SD, Chen WD, Pretlow TP, Yang B, Akiyama Y, Van Engeland M, et al. Epigenetic inactivation of SFRP genes allows constitutive WNT signaling in colorectal cancer. Nat Genet. 2004;36:417–422. doi: 10.1038/ng1330. [DOI] [PubMed] [Google Scholar]
- 18.Suzuki H, Toyota M, Nojima M, Mori M, Imai K. SFRP, a family of new colorectal tumor suppressor candidate genes. Nippon Rinsho. 2005;63:707–719. [PubMed] [Google Scholar]
- 19.Rattner A, Hsieh JC, Smallwood PM, Gilbert DJ, Copeland NG, Jenkins NA, Nathans J. A family of secreted proteins contains homology to the cysteine-rich ligand-binding domain of frizzled receptors. Proc Natl Acad Sci USA. 1997;94:2859–2863. doi: 10.1073/pnas.94.7.2859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Qi J, Zhu YQ, Luo J, Tao WH. Hypermethylation and expression regulation of secreted frizzled-related protein genes in colorectal tumor. World J Gastroenterol. 2006;12:7113–7117. doi: 10.3748/wjg.v12.i44.7113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Eads CA, Danenberg KD, Kawakami K, Saltz LB, Blake C, Shibata D, Danenberg PV, Laird PW. MethyLight: a high-throughput assay to measure DNA methylation. Nucleic Acids Res. 2000;28:E32. doi: 10.1093/nar/28.8.e32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Eads CA, Lord RV, Wickramasinghe K, Long TI, Kurumboor SK, Bernstein L, Peters JH, DeMeester SR, DeMeester TR, Skinner KA, et al. Epigenetic patterns in the progression of esophageal adenocarcinoma. Cancer Res. 2001;61:3410–3418. [PubMed] [Google Scholar]
- 23.Zou H, Molina JR, Harrington JJ, Osborn NK, Klatt KK, Romero Y, Burgart LJ, Ahlquist DA. Aberrant methylation of secreted frizzled-related protein genes in esophageal adenocarcinoma and Barrett’s esophagus. Int J Cancer. 2005;116:584–591. doi: 10.1002/ijc.21045. [DOI] [PubMed] [Google Scholar]
- 24.Jass JR. Hyperplastic polyps and colorectal cancer: is there a link? Clin Gastroenterol Hepatol. 2004;2:1–8. doi: 10.1016/s1542-3565(03)00284-2. [DOI] [PubMed] [Google Scholar]
- 25.Speake D, Biyani D, Frizelle FA, Watson AJ. Flat adenomas. ANZ J Surg. 2007;77:4–8. doi: 10.1111/j.1445-2197.2006.03847.x. [DOI] [PubMed] [Google Scholar]
- 26.Buecher B, Bezieau S, Dufilhol C, Cauchin E, Heymann MF, Mosnier JF. Emerging concepts in colorectal serrated polyps. Gastroenterol Clin Biol. 2007;31:39–54. doi: 10.1016/s0399-8320(07)89325-0. [DOI] [PubMed] [Google Scholar]
- 27.Souglakos J. Genetic alterations in sporadic and hereditary colorectal cancer: implementations for screening and follow-up. Dig Dis. 2007;25:9–19. doi: 10.1159/000099166. [DOI] [PubMed] [Google Scholar]
- 28.Kim YH, Petko Z, Dzieciatkowski S, Lin L, Ghiassi M, Stain S, Chapman WC, Washington MK, Willis J, Markowitz SD, et al. CpG island methylation of genes accumulates during the adenoma progression step of the multistep pathogenesis of colorectal cancer. Genes Chromosomes Cancer. 2006;45:781–789. doi: 10.1002/gcc.20341. [DOI] [PubMed] [Google Scholar]
- 29.Chan AO, Issa JP, Morris JS, Hamilton SR, Rashid A. Concordant CpG island methylation in hyperplastic polyposis. Am J Pathol. 2002;160:529–536. doi: 10.1016/S0002-9440(10)64872-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kaz A, Kim YH, Dzieciatkowski S, Lynch H, Watson P, Kay Washington M, Lin L, Grady WM. Evidence for the role of aberrant DNA methylation in the pathogenesis of Lynch syndrome adenomas. Int J Cancer. 2007;120:1922–1929. doi: 10.1002/ijc.22544. [DOI] [PubMed] [Google Scholar]
- 31.Derks S, Postma C, Moerkerk PT, van den Bosch SM, Carvalho B, Hermsen MA, Giaretti W, Herman JG, Weijenberg MP, de Bruine AP, et al. Promoter methylation precedes chromosomal alterations in colorectal cancer development. Cell Oncol. 2006;28:247–257. doi: 10.1155/2006/846251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zitt M, Zitt M, Muller HM. DNA methylation in colorectal cancer--impact on screening and therapy monitoring modalities? Dis Markers. 2007;23:51–71. doi: 10.1155/2007/891967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Huang Z, Li L, Wang J. Hypermethylation of SFRP2 as a potential marker for stool-based detection of colorectal cancer and precancerous lesions. Dig Dis Sci. 2007;52:2287–2291. doi: 10.1007/s10620-007-9755-y. [DOI] [PubMed] [Google Scholar]
- 34.Lenhard K, Bommer GT, Asutay S, Schauer R, Brabletz T, Goke B, Lamerz R, Kolligs FT. Analysis of promoter methylation in stool: a novel method for the detection of colorectal cancer. Clin Gastroenterol Hepatol. 2005;3:142–149. doi: 10.1016/s1542-3565(04)00624-x. [DOI] [PubMed] [Google Scholar]
- 35.Cong F, Zhang J, Pao W, Zhou P, Varmus H. A protein knockdown strategy to study the function of beta-catenin in tumorigenesis. BMC Mol Biol. 2003;4:10. doi: 10.1186/1471-2199-4-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kim HC, Roh SA, Ga IH, Kim JS, Yu CS, Kim JC. CpG island methylation as an early event during adenoma progression in carcinogenesis of sporadic colorectal cancer. J Gastroenterol Hepatol. 2005;20:1920–1926. doi: 10.1111/j.1440-1746.2005.03943.x. [DOI] [PubMed] [Google Scholar]
- 37.Ogino S, Kawasaki T, Brahmandam M, Cantor M, Kirkner GJ, Spiegelman D, Makrigiorgos GM, Weisenberger DJ, Laird PW, Loda M, et al. Precision and performance characteristics of bisulfite conversion and real-time PCR (MethyLight) for quantitative DNA methylation analysis. J Mol Diagn. 2006;8:209–217. doi: 10.2353/jmoldx.2006.050135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Weisenberger DJ, Campan M, Long TI, Kim M, Woods C, Fiala E, Ehrlich M, Laird PW. Analysis of repetitive element DNA methylation by MethyLight. Nucleic Acids Res. 2005;33:6823–6836. doi: 10.1093/nar/gki987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Trinh BN, Long TI, Laird PW. DNA methylation analysis by MethyLight technology. Methods. 2001;25:456–462. doi: 10.1006/meth.2001.1268. [DOI] [PubMed] [Google Scholar]
- 40.Wynter CV, Kambara T, Walsh MD, Leggett BA, Young J, Jass JR. DNA methylation patterns in adenomas from FAP, multiple adenoma and sporadic colorectal carcinoma patients. Int J Cancer. 2006;118:907–915. doi: 10.1002/ijc.21363. [DOI] [PubMed] [Google Scholar]
- 41.Garber K. New gene discoveries may boost DNA stool testing for colorectal cancer. J Natl Cancer Inst. 2004;96:820–821. doi: 10.1093/jnci/96.11.820. [DOI] [PubMed] [Google Scholar]
- 42.McLoughlin RM, O’Morain CA. Colorectal cancer screening. World J Gastroenterol. 2006;12:6747–6750. doi: 10.3748/wjg.v12.i42.6747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Halligan S, Taylor SA. CT colonography: results and limitations. Eur J Radiol. 2007;61:400–408. doi: 10.1016/j.ejrad.2006.07.030. [DOI] [PubMed] [Google Scholar]