Scheme 15.
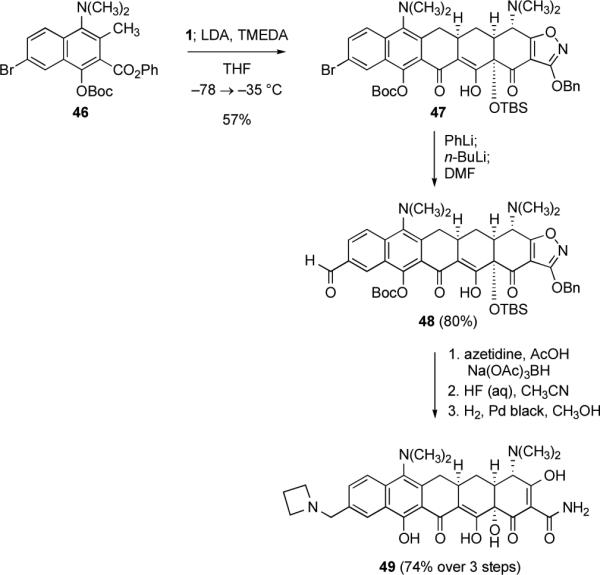
Synthesis of the diversifiable pentacycline precursors 47 and 48 by Michael–Claisen cyclization using the phenyl naphthoate ester 46 and enone 1 as substrates, with deprotonation in situ. Further transformation of 48 by a reductive amination reaction provides a route to 7-dimethylamino-alkylaminomethylpentacyclines, as illustrated by the synthesis of the 7-dimethylamino-azetidinylmethylpentacycline 49.
