Table 2.
Synthesis of 6-substituted tetracyclines by Michael—Claisen cyclization using D-ring precursors with anion-stabilizing substituents in the benzylic position and enone 1 as substrates, with deprotonation in situ, followed by deprotection [(i) HF (aq), CH3CN; (ii) H2, Pd/C, CH3OH-dioxane].37
| D-ring Precursor | Cyclization Conditions | Cyclization Product | Cyclization Yield | Tetracycline | Deprotection Yield |
|---|---|---|---|---|---|
 |
1; LDA −78 → −10 °C |
 |
58% |  |
58% |
 |
1; LDA −78 → −10 °C |
 |
72% |  |
60% |
 |
1; LDA −78 → −10 °C |
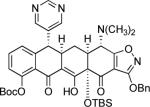 |
44% |  |
23% |
 |
1; LDA −78 → −10 °C |
 |
83% |  |
98% |
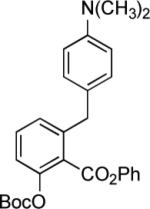 |
1; LDA, TMEDA −78 → −10 °C |
 |
25% |  |
64% |
 |
1; LHMDS −78 → −10 °C |
 |
72% |  |
43% |
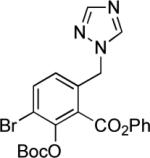 |
1; LHMDS −78 → −10 °C |
 |
38% |  |
65% |
