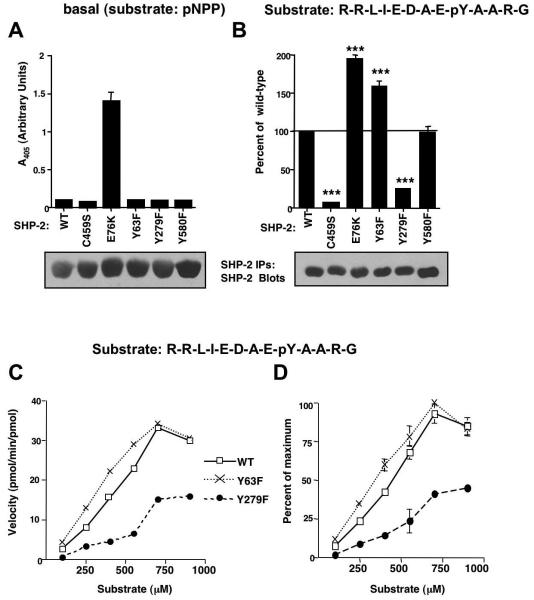
Figure 4. SHP-2 phosphorylation mutants have altered activities towards phosphotyrosine-containing substrates.
(A) Wild-type or mutant forms of SHP-2 were immunoprecipitated from serum-starved, overexpressing 293T cells, incubated with pNPP substrate and supernatant A405 was measured. Some error bars are too small to be visualized. (B) Wild-type or mutant forms of SHP-2 were immunoprecipitated from serum-starved overexpressing 293T cells, incubated with 250 μM substrate (R-R-L-I-E-D-A-E-pY-A-A-R-G) for 30 minutes at 37°C, and phosphate release into the supernatant was quantitated by incubation with Malachite Green, and measurement of A620. Absorbance values were compared to a standard phosphate curve to determine pmoles of phosphate released. Values obtained for mutant forms of SHP-2 were expressed as a percentage of wild-type with mean ± s.e.m. from three independent experiments. ***p≤0.001 using one-way ANOVAs followed by Bonferroni post-hoc tests. Immunoprecipitates were blotted with SHP-2 antibody (representative experiments are shown). (C) SHP-2 immunoprecipitates from expressing 293T cells (B) were incubated in a phosphatase assay with increasing concentrations of peptide substrate. Velocity values were converted to pmol/min/pmol of enzyme utilized. A representative experiment is shown. (D) Velocity values from two independent experiments (C) were standardized by converting the values to percent of the maximum obtained in each experiment (mean ± s.e.m.). Some error bars are too small to be visualized.