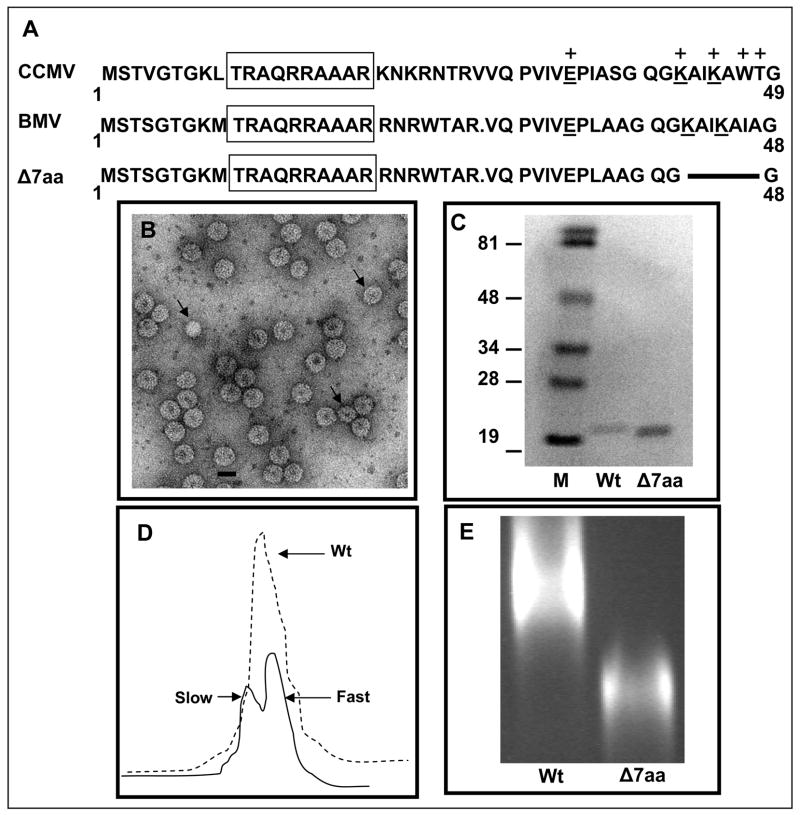
Figure 1.
Characteristic features of BMV CP variant Δ7aa. (A) Sequence alignment of the N-terminal amino acids of CCMV and BMV CP. Within this region, the sequences of BMV and CCMV are highly homologous. BMV CP is one amino acid residue shorter than that of CCMV due to a deletion at position 27. The boxed region indicates the N-terminal arginine rich motif (N-ARM). Residues of CCMV CP involved in RNA binding are indicated by (+). The conserved residues involved in RNA binding are underlined. The extent of the engineered deletion in variant Δ7aa is shown by the solid line. (B) Electron micrograph of purified virions of Δ7aa. Purified virions were subjected negative staining prior to viewing under EM. The smaller size particles are indicated by the arrow head. Bar = 20 nm. (C) Western blot analysis of wt and Δ7aa CP. Purified virions of wt and Δ7aa were suspended in SDS-PAGE sample buffer, denatured by boiling the sample and subjected to 16% SDS-PAGE. After transferring the fractionated proteins to a nitrocellulose membrane, the blot was probed with antibodies specific for BMV. Note the difference in mobility of CP for wt and Δ7aa. M, marker lane. Sizes of protein markers in kDa are shown to the left. (D) Sucrose density gradient sedimentation analysis of partially purified virions of Δ7aa. Partially purified virions were subjected to centrifugation through 10%–40% linear sucrose density gradient. Wt BMV virions sedimenting as a single peak is indicated by the dotted line while those of Δ7aa sedimenting as slow and fast peaks by the solid line. (E) Virion mobility profiles of wt and Δ7aa. Purified virions of wt and Δ7aa were electrophoresed in a 1% agarose/TAE gel containing EtBr. Notice the mobility of Δ7aa virions is different from that of wt.