Abstract
The developmental competence of in vitro-matured (IVM) rhesus macaque cumulus oocyte complexes (COCs) is deficient compared with in vivo-matured (IVM) oocytes. To improve oocyte quality and subsequent embryo development following IVM, culture conditions must be optimized. A series of experiments was undertaken to determine the role of epidermal growth factor (EGF) during IVM of rhesus macaque COCs. The addition of Tyrphostin AG-1478 (a selective inhibitor of the EGF receptor EGFR) to the IVM medium yielded fewer oocytes maturing to metaphase II of meiosis II (MII), decreased cumulus expansion, and a lower percentage of embryos that developed to the blastocyst stage compared with untreated IVM controls, indicating that EGFR activation is important for IVM maturation in the rhesus macaque. However, the addition of recombinant human EGF (r-hEGF) to the IVM medium did not enhance outcome. The expression of mRNAs encoding the EGF-like factors amphiregulin, epiregulin, and betacellulin in cumulus cells indicates that these factors produced by cumulus cells may be responsible for maximal EGFR activation during oocyte maturation, precluding any further effect of exogenous r-hEGF. Additionally, these results illustrate the potential futility of exogenous supplementation of IVM medium without prior knowledge of pathway activity.
Keywords: rhesus monkey, follicle, ovary, gene expression, epidermal growth factor
the ability of primate oocytes to complete nuclear and cytoplasmic in vitro maturation (IVM) is markedly inferior to that of oocytes from other species (17, 24, 52, 53, 64, 69, 72) and yields oocytes of much lesser quality compared with primate oocytes matured in vivo (2, 4, 44, 63, 67, 68, 79). Until a few years ago, there were only two reports of rhesus monkey oocytes matured in vitro from the germinal vesicle stage and subsequently able to support blastocyst formation after in vitro fertilization and embryo culture (63, 64). There is still only one report of live offspring born as a result of these techniques (66). All of these experiments used immature oocytes from monkeys given 7 days of human recombinant follicle stimulating hormone (7-day FSH primed). Oocytes from nonstimulated macaques have fared even more poorly and yielded only a few embryos that developed to the blastocyst stage (65, 80, 84).
Although IVM oocytes may complete nuclear maturation and become able to be fertilized, many fail to acquire developmental competence as measured by the ability to support embryogenesis (67). This is generally attributed to poor oocyte quality or inadequate cytoplasmic maturation (8, 43, 51, 76). The precise molecular definition of these characterizations remains unknown, although it is widely accepted that improvement in oocyte quality and subsequent embryo development may be achieved by providing an IVM environment that is more supportive of maturation.
In the follicle, a wide array of growth factors, signaling molecules, and nutrients surround the oocyte and support maturation and developmental competence of oocytes (26, 34). Bidirectional communication between the oocyte and surrounding cumulus and granulosa cells is essential for developmental competence (5, 19, 20, 41). Oocyte-secreted factors such as GDF-9 and BMP-15 play an essential role in folliculogenesis and oocyte development (25, 74). Although the nature of oocyte-cumulus cell interactions has been extensively studied in some models, such knowledge of nonhuman primate models is very limited. Available data indicate that primate oocyte maturation may differ in significant ways from nonprimate species (45, 85). Hence, extensive research in the nonhuman primate is needed to elucidate molecular differences between oocytes and cumulus cells matured in vitro and in vivo, along with key signaling molecules necessary for maturation. Studies using nonhuman primate cumulus oocyte complexes (COCs) are confounded by the potential for paracrine factors, signaling molecules, and growth factors to be secreted by cumulus cells. Adding growth factors that are beneficial to oocyte maturation in some species may not prove useful in other species if these factors are already secreted locally by cumulus cells.
Epidermal growth factor (EGF) is one member of a large family of closely related proteins that includes amphiregulin (AREG), epiregulin (EREG), betacellulin (BTC), and transforming growth factor-α (TGFα). All of these EGF-like ligands use the same EGF receptor (EGFR) (70). In general, EGF and EGF-like ligands are associated with cell growth, proliferation, and differentiation. EGF-like ligands enhance expansion of the COC (31, 37–39, 49, 54), promote steroidogenesis (33, 70), and improve IVM of oocytes in a variety of species: mouse (14, 16), cow (13, 37, 38), rabbit (39), human (28, 29), sheep (30), rat (15), and pig (11, 12). EGF studies in the human utilized naked oocytes or oocytes from nonstimulated ovaries for IVM (28, 29). Any action of EGF on the COC may be mediated by changes in expression of cell cycle-related genes, namely cyclins and cyclin-dependent kinases (CDKs). In rats, LH/hCG (human chorionic gonadotropin) induces additional cell cycle progression in cumulus cells, which may be via cyclin E/CDK2 (7, 60). The effect of EGF on IVM of rhesus macaque oocytes has not been investigated. We report here that the EGFR signaling pathway is active and necessary for rhesus monkey IVM, that the mRNAs encoding EGF-like ligands are expressed in the cumulus cells, but that exogenous EGF supplementation during IVM does not enhance IVM outcome beyond what is promoted by endogenous factors.
MATERIALS AND METHODS
Animals.
Adult female rhesus macaques (Macaca mulatta) were housed at the California National Primate Research Center (CNPRC) as described previously (47). Only females with a history of normal menstrual cycles were selected for this study. All procedures for maintenance and handling of the animals were reviewed and approved in advance by the Institutional Animal Use and Care Administrative Advisory Committee at the University of California at Davis. Females were observed daily for signs of vaginal bleeding, and the first day of menses was assigned cycle day 1. Starting on cycle day 1–4 recombinant macaque FSH (r-mFSH; National Hormone and Peptide Program, UCLA, Los angeles, CA) was administered (37.5 IU) twice daily intramuscularly for 7 days total. Antide (Ares-Serono, Randolph, MA) was administered subcutaneously (0.5 mg/kg body wt) once daily in the morning on the last 3 days when r-mFSH was given to prevent an endogenous LH surge. Immature oocytes were collected on the morning following the last dose of r-mFSH. COCs were aspirated by ultrasound-guided oocyte collection (77, 78) and retrieved from aspirates as previously described (47).
In vitro maturation of COCs with or without an EGFR pathway inhibitor.
Retrieved immature COCs were randomly placed into 70-μl drops of medium from three different treatment groups: control, vehicle A, or Tyrphostin AG-1478 (selective inhibitor of EGFR protein; Sigma-Aldrich, St. Louis, MO). AG-1478 was reconstituted in 100% DMSO. The control treatment group consisted of M1A medium (46, 67). The vehicle A treatment group consisted of M1A with 0.1% DMSO. The AG-1478 treatment group consisted of M1A with 10 μM AG-1478 (0.1% DMSO). COCs were incubated in a humidified atmosphere of 5% CO2 in air for 28–30 h at 37°C. Following incubation, all COCs were prepared for in vitro fertilization (IVF) and inseminated.
IVF and embryo development.
COCs were inseminated after 28–30 h of IVM. All COCs were inseminated regardless of nuclear status. Oocytes were rinsed and transferred into Tyrode's lactate containing 0.1 mg/ml polyvinyl alcohol (TL-PVA) medium (37°C) under oil and inseminated according to standard procedure for IVF of rhesus macaque oocytes (78). Semen was collected from male macaques that had been trained for this procedure as previously reported (61). Sperm were washed from seminal plasma and resuspended in TL-BSA medium (4). The next morning, oocytes were transferred into 70-μl drops of chemically defined, protein-free hamster embryo culture medium 9 (HECM-9) under oil (37°C) and incubated at 37°C in a humidified atmosphere of 5% CO2-10% O2-85% N2 for 48 h (42). On day 3 (60 h postinsemination), noncleaved oocytes were assessed for nuclear maturation. Noncleaved oocytes exhibiting a polar body and all embryos were classified as having matured to metaphase II of meiosis II (MII). Embryos were transferred into 70-μl drops of HECM-9 medium with 5% bovine calf serum (Gemini Bioproducts, West Sacramento, CA) under mineral oil and incubated as described above. Embryos were transferred to fresh medium every other day until no further development was observed. When development ceased, the percentage of embryos developing to the blastocyst stage was calculated for each treatment.
Blastocyst stage embryos were fixed and stained for differential cell counting with Oct 3/4 as previously described (47). Optical sectioning was performed with a Delta Vision microscope using a × 20, Olympus UApo 340/0.70 water immersion objective (Olympus Optical, Tokyo, Japan). A z-projection was created for each blastocyst stage embryo. Images were coded and read blindly. Manual cell counts were taken of the inner cell mass (ICM) and trophectoderm (TE) cells.
Cumulus cell expansion.
Cumulus expansion was measured with images of the COCs taken before and after the 28- to 30-h IVM incubation, as previously described (47). Replicate COCs within each treatment group were averaged for each female. Percent increase in cumulus expansion was found by dividing the difference between the average COC area before and after incubation by the COC area before incubation. COCs from six females were used for cumulus expansion experiments (114 COCs total).
In vitro maturation of oocytes with or without EGF.
Immature COCs were randomly placed into 70 μl drops of medium from one of three different treatment groups: control, vehicle B or EGF. Recombinant human EGF (r-hEGF; R&D Systems, Minneapolis, MN) was reconstituted in 10 mM acetic acid (Sigma-Aldrich, St. Louis, MO) with 1% BCS (Gemini Bioproducts, West Sacramento, CA) in water. The control treatment group consisted of M1A medium alone. The vehicle B treatment group consisted of M1A with a final concentration of 5 μM acetic acid. The EGF treatment group consisted of M1A supplemented with 100 ng/ml r-hEGF (5 μM acetic acid). A dose response experiment was not carried out with r-hEGF due to limited resources. While 100 ng/ml is higher than used in the literature (2–50 ng/ml) (12–14, 29, 30, 38) there are multiple EGFR ligands in follicular fluid and so the EGF activity is probably very high. While the optimal EGF concentration for humans is unknown a dose response experiment in the cow found doses between 1 and 100 ng/ml to stimulate cumulus expansion and nuclear maturation (37). COCs were incubated in a humidified atmosphere of 5% CO2 in air for 28–30 h at 37°C. Following incubation, COCs were either fixed for assessment of nuclear maturation and cytoskeletal elements or prepared for in vitro fertilization (IVF). Embryo culture after IVF was performed as described above. Nuclear maturation after IVF was assessed 60 h postinsemination, and the percentage of blastocyst stage embryos was calculated when development ceased.
Staining for nuclear status, cytoplasmic microtubules of oocytes, transzonal processes of cumulus cells, and gap junctions.
Nuclear status, cytoskeletal elements, and gap junctions were assessed by immunostaining oocytes for tubulin, actin, nuclear material, and connexin 43 (1). A total of 36 COCs were stained from four females (11–13 COCs per treatment group). Intact cumulus layers were observed in all COCs used. COCs were fixed in a microtubule stabilizing buffer for 1 h and placed in blocking solution overnight at 4°C (1). All COCs were transferred from blocking solution into 30-μl drops of Dulbecco's phosphate-buffered saline (DPBS; Sigma, St. Louis, MO) on separate 10-well slides (PolySciences, Warrington, PA). Washes were performed as previously described (47). All COCs were washed (3×) with DPBS. COCs were incubated in a 37°C, humid environment for 2–4 h with monoclonal rat anti-α-tubulin antibody, monoclonal mouse anti-β-tubulin antibody (Accurate Scientific, Westbury, CT), and rabbit anti-connexin 43 (Sigma-Aldrich, St. Louis, MO). After a wash in DPBS (3×), COCs were incubated again for 1 h with Alexa 555 goat anti-mouse IgG conjugate, Alexa 555 goat anti-rat IgG conjugate, Alexa 635 goat anti-rabbit IgG, and Alexa 488-phalloidin (Invitrogen, Carlsbad, CA). COCs were washed with DPBS (3×) and mounted in Vectashield with DAPI (Vector Laboratories, Burlingame, CA) and stored at 4°C in the dark. Nuclear maturation, cytoskeletal elements, and transzonal processes were examined and imaged as previously described (46). Gap junctions were imaged in the same manner as transzonal processes and were manually counted in each z-projection.
Immunocytochemistry of EGFR and phosphorylated EGFR.
All COCs used for immunocytochemistry were supernumerary COCs obtained from control cohorts of unrelated studies. Preliminary studies stained COCs from FSH-primed ovaries (25 COCs from 5 females), FSH-primed ovaries and 24-h IVM culture (2 COCs from 2 females), and FSH- and hCG-primed ovaries (7 COCs from 4 females) for EGFR. COCs were fixed, blocked, and washed as described above. Staining for EGFR was performed by incubating COCs in a humidified chamber for 3–4 h at 37°C with rabbit polyclonal anti-EGFR (Abcam, Cambridge, MA). COCs were washed (3×) with DPBS and incubated at 37°C for 1–2 h in a humidified environment with Alexa fluor 635 or 555 goat anti-rabbit IgG (Invitrogen, Carlsbad, CA). Alexa 488-conjugated phalloidin (Invitrogen) was added to the secondary antibody solution to allow for the visualization of f-actin. COCs were washed in DPBS (3×), mounted in Vectashield with DAPI, and stored at 4°C in the dark.
Staining for phosphorylated (p)EGFR was performed on COCs from FSH-primed ovaries (6 COCs from 3 females), FSH-primed ovaries and 24-h IVM culture (6 COCs from 3 females), and oocytes that were matured in vivo (6 COCs from 3 females). COCs from FSH-primed ovaries were used as negative controls (2 COCs from 2 females). COCs were fixed, blocked, and washed as described above. Staining for pEGFR was performed by incubating COCs in a humidified chamber overnight at 4°C with rabbit polyclonal anti-pEGFR (Millipore, Billerica, MA) diluted 1:100 in DPBS. The primary antibody was substituted with nonimmune fetal bovine serum (Gemini Bioproducts, West Sacramento, CA) diluted 1:100 in DPBS for negative controls. COCs were washed (3×) with DPBS and incubated at 37°C for 2 h in a humidified environment with Alexa fluor 555 goat anti-rabbit IgG diluted 1:100 in DPBS. Alexa 488-conjugated phalloidin was added to the secondary antibody solution to allow for the visualization of f-actin. COCs were washed in DPBS (3×), mounted in Vectashield with DAPI, and stored at 4°C in the dark. COCs stained for EGFR and pEGFR were imaged with a Zeiss LSM 510 confocal laser scanning microscope with a Zeiss Plan-Apochromat, ×63/1.40 oil immersion objective for fluorescence (Carl Zeiss, Jena, Germany). 30–40 z-sections of 0.5 μm were collected for each oocyte, and a z-projection was created. The images were analyzed using Adobe Photoshop (Adobe Systems, San Jose, CA). Negative control images were used to determine the appropriate level for thresholding.
Cumulus and granulosa cell collection for real-time qRT-PCR.
Immature COCs and granulosa cells were collected on the morning following the last dose of r-mFSH for in vitro maturation (IVM). For collection of in vivo-matured oocytes (VVM), females were injected intramuscularly with r-hCG (1,000 IU Ovidrel; Serono, Rockland, MA) on treatment day 8 in addition to the FSH treatment outlined above. Follicular contents were aspirated 28–30 h following hCG, as described above. Cumulus and granulosa cells collected after r-mFSH stimulation will be referred to as IVM samples, and cumulus and granulosa cells collected after r-mFSH and hCG stimulation will be referred to as VVM samples. At the time of aspiration, IVM or VVM granulosa cells were collected in TL-HEPES medium (37°C) containing 0.1 mg/ml PVA (4) and 5 ng/ml r-hFSH (Organon, Roseland, NJ). Granulosa cells were separated from blood as previously described (23, 73). The final suspension (20 μl) was placed in a 1.5-ml tube and centrifuged at 6,000 g for 5 min (∼88,000–364,000 cells). After supernatant removal, the cell pellet was suspended in 20 μl of PicoPure extraction buffer (MDS Analytical Technologies, Sunnyvale, CA) and stored at −80°C.
Retrieved immature COCs (IVM) were randomly placed into 70-μl treatment drops of M1A medium and incubated in a humidified atmosphere of 5% CO2 in air for 28–30 h at 37°C. Retrieved in vivo-matured COCs (VVM) and IVM COCs after incubation were placed in 10 mg/ml hyaluronidase (MP Biomedicals, Solon, OH) in TL-PVA that was preequilibrated to 37°C. COCs were stripped of cumulus cells using a Stripper (MidAtlantic Diagnostics, Mount Laurel, NJ). Oocytes were removed (for other studies), and ∼50–100 cumulus cells in the remaining volume were placed into a 1.5-ml tube and centrifuged at 6,000 g for 5 min. After supernatant removal, the cell pellet was suspended in 20 μl of PicoPure extraction buffer and stored at −80°C. Granulosa and cumulus cell samples were shipped overnight on dry ice to Temple University (Philadelphia, PA), where they were added to the Primate Embryo Gene Expression Resource (PREGER) (www.preger.org) (81–83).
Real-time qRT-PCR analysis.
Total RNA was isolated from cumulus or granulosa cells with the PicoPure RNA extraction kit (MDS Analytical Technologies, Sunnyvale, CA) according to the manufacturer's protocols. Reverse transcription and whole transcriptome amplification for individual samples were achieved with the QuantiTect whole transcriptome kit (Qiagen, Valencia, CA) using 50 ng of total RNA per sample. TaqMan gene expression assays were custom designed on the basis of rhesus cDNA sequences and performed with reagents manufactured and validated by Applied Biosystems (Foster City, CA). Real-time quantitative PCR was performed with an ABI Prism 7000 Sequence Detection System. For each target gene, nine replicates of VVM cumulus cells, five replicates of IVM cumulus cells, six replicates of VVM granulosa cells, and five replicates of IVM granulosa cells were used for the quantitative gene expression assay. Reactions receiving rhesus testis cDNA or no-input template served as positive and a negative controls, respectively. The relative level of expression of each target mRNA was normalized to the endogenous 18S ribosomal RNA, and the relative mRNA abundance ratio of IVM to VVM groups was calculated using the comparative CT method as recommended by the manufacturer (ABI 7300/7500 Technical Bulletin, Applied Biosystems) (36).
Statistical analysis.
The percentage of COCs maturing to MII, percentage of embryos developing to the blastocyst stage, actin and tubulin transzonal processes, number of gap junctions, and average percent increase in cumulus expansion were analyzed with a repeated-measures one-way ANOVA. The Tukey-Kramer posttest was used when P < 0.05. Values are expressed as means ± SE per female, and n represents the number of females used for each experiment. Differential cell counts of blastocyst stage embryos were compared between treatment groups with a one-way ANOVA, and n represents the number of blastocyst stage embryos. Statistics were run with Prism software (GraphPad Software, San Diego, CA). COC cytoplasmic microtubule scores from various treatment groups were compared using an extension of the Mantel-Haenszel χ2 statistic (mean score test), using P < 0.05 as the significance level. Due to small sample size per cell in the microtubule score data set, a Monte Carlo simulation of Fisher's exact test was also performed (exact test for mean score statistic unavailable). Statistics were run with SAS version 9.1 (SAS Institute, Cary, NC). Statistical evaluation for quantitative PCR was performed by groupwise comparison between VVM and IVM groups, using the Relative Expression Software tool (50).
RESULTS
The EGFR pathway is active and essential for IVM.
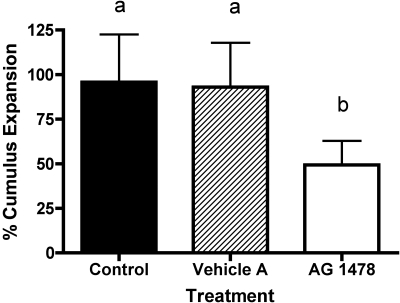
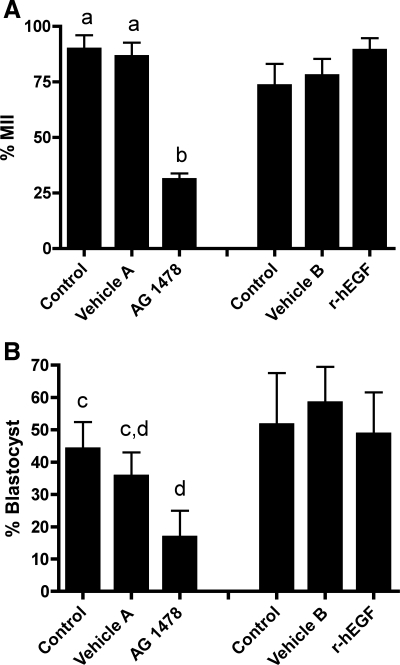
Studies in other species indicate that EGF and EGF-like ligands can exert beneficial effects during IVM (11–13, 15, 16, 27, 29, 30, 37–39). To test whether EGFR signaling might likewise contribute to IVM in the rhesus monkey, we undertook two series of IVM studies. In the first series, we treated rhesus monkey COCs with an EGFR pathway inhibitor, AG-1478. Comparisons between untreated, vehicle-treated, and AG-1478-treated COCs revealed potent effects of the inhibitor on IVM. Cumulus cell expansion was lower in COCs matured in the presence of AG-1478 compared with the control and vehicle A treatment groups (Fig. 1; P < 0.05). The addition of AG-1478 to IVM medium significantly decreased the percentage of oocytes maturing to MII compared with the control and vehicle A treatment group (Fig. 2A; P < 0.001). The percentage of embryos developing to the blastocyst stage was lower in the AG-1478 treatment group compared with the untreated controls (P = 0.0198) but not significantly different from vehicle A (Fig. 2B). The number of cells in the ICM of blastocyst stage embryos was significantly lower in the AG-1478 compared with the vehicle A treatment group (P = 0.0149, Table 1, experiment 1). No other differential cell counts of blastocyst stage embryos differed between treatment groups (Table 1, experiment 1). These results indicate that AG-1478 significantly inhibited oocyte maturation and that the EGFR pathway is therefore active and contributes to IVM. A broader effect on oocyte quality exists, seen as a significant reduction in the allocation of cells to the ICM and a trend toward reduced blastocyst formation.
Fig. 1.
Average percent increase in cumulus expansion after 28–30 h in in vitro-matured (IVM) medium supplemented with or without AG-1478. Each bar represents mean ± SE (n = 6 females). Significant differences between groups are indicated by different letters (P < 0.05).
Fig. 2.
Average percent nuclear maturation 60 h postinsemination (A) and subsequent development of embryos to the blastocyst stage (B). Each bar represents mean ± SE. Significant differences are indicated by different letters (a,bP < 0.001; c,dP < 0.05).
Table 1.
Differential cell counts of blastocyst stage embryos
| Experiment | Treatment | No. of Blastocysts | Total Cell Count | ICM | ICM/TE, % |
|---|---|---|---|---|---|
| 1 | Control | 7 | 237.1±16.3 | 31.3±4.4 | 15.3±2.1 |
| Vehicle A | 9 | 250.3±36.9 | 36.9±3.9a | 19.2±2.1 | |
| AG-1478 | 4 | 184.3±36.8 | 15.3±3.3b | 10.6±2.9 | |
| 2 | Control | 8 | 159.0±21.4 | 11.3±1.2 | 8.5±1.3 |
| Vehicle B | 8 | 178.0±17.7 | 13.1±0.7 | 8.5±0.8 | |
| r-hEGF | 8 | 154.0±19.0 | 14.8±2.8 | 11.7±2.8 |
Values are means ± SE. r-hEGF, recombinant human epidermal growth factor; ICM, inner cell mass; TE, trophectoderm.
Different subscripts within column indicate significant difference, P < 0.05.
Effect of exogenous EGF ligand on rhesus monkey IVM.
Having suggested that the EGFR pathway is active and necessary for rhesus monkey IVM, we next wanted to determine whether this pathway is maximally active or whether exogenous EGF can enhance the success of IVM further. We therefore compared results for untreated, vehicle-treated, and r-hEGF-treated COCs. Nuclear maturation rates (%MII) after 28–30 h of IVM did not change between control, vehicle B, and r-hEGF treatment groups (22.9 ± 15.7, 16.7 ± 9.6, and 18.3 ± 10.7, respectively). Nuclear maturation rates 60 h postinsemination, percentage of embryos developing to the blastocyst stage, and differential cell counts of blastocyst stage embryos did not change with the addition of r-hEGF to IVM medium (Fig. 2, A and B; Table 1, experiment 2). No difference was found in the number of actin and tubulin transzonal processes, number of gap junctions (Table 2), or cytoplasmic microtubule scores (Table 3) between the control, vehicle B, and r-hEGF treatment groups in COCs fixed after 28–30 h of IVM.
Table 2.
Average number of COC actin and tubulin transzonal processes and gap junctions per female
| Treatment | No. of Females | Total Oocytes | Actin | Tubulin | Gap Junctions |
|---|---|---|---|---|---|
| Control | 4 | 12 | 37.8±10.0 | 12.9±2.9 | 25.5±4.1 |
| Vehicle B | 4 | 11 | 52.9±14.2 | 22.3±5.8 | 26.3±4.7 |
| r-hEGF | 4 | 13 | 56.7±16.1 | 12.8±3.6 | 31.9±2.5 |
Values are means ± SE. COC, cumulus oocyte complexes.
Table 3.
Contingency table of oocytes cross-categorized according to in vitro maturation treatment and microtubule scores
| Treatment | No. of Females | Total Oocytes |
Microtubule Scores |
||||
|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | |||
| Control | 4 | 12 | 83.33 | 16.67 | 0 | 0 | 0 |
| Vehicle B | 4 | 11 | 81.82 | 18.18 | 0 | 0 | 0 |
| r-hEGF | 4 | 13 | 69.23 | 15.38 | 7.69 | 0.00 | 7.69 |
Percentage of oocytes within each row are indicated.
EGFR phosphorylation in COCs.
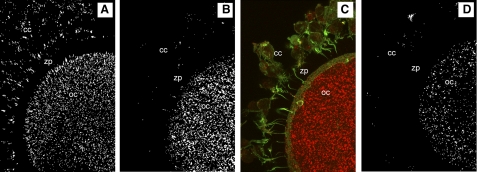
Preliminary studies revealed EGFR staining on the surface of rhesus macaque cumulus cells and within the oocyte cytoplasm (data not shown). pEGFR also labeled positive on the surface of cumulus cells and within the oocyte cytoplasm (Fig. 3, A–D). Labeling was absent in cumulus cell cytoplasm and transzonal processes. The pattern of EGFR and pEGFR labeling was similar in all COCs and did not appear to change over time (Fig. 3, A–D). Slight differences in staining intensity were observed between oocytes, but no difference in intensity was observed between different groups. Microarray data for macaque in vitro- and in vivo-matured oocytes obtained previously are presented in Table 4 (35). The array data showed some detection of EGFR in macaque oocyte samples, but this detection was inconsistent and too low to pass the assigned threshold for “present” call for a number of the samples. EGFR expression was detected in macaque cumulus and granulosa cells via qPCR (Table 5).
Fig. 3.
Cumulus oocyte complexes (COCs) stained for phosphorylated epidermal growth factor receptor (pEGFR). A: COC from FSH-primed ovary. B: COC from FSH-primed ovary and fixed after 28–30 h of IVM. C: merged image of B showing intact cumulus cells (green, f-actin; red, pEGFR), D: COC from FSH- and human chorionic gonadotropin (hCG)-primed ovary. A–D: pEGFR labeled positive in cumulus cells (cc) and in the oocyte cytoplasm (oc) of all COCs stained. The zona pellucida (zp) was negative for labeling.
Table 4.
Gene expression profiles from cDNA microarray analysis of IVM and VVM rhesus MII stage oocytes*
| Gene Symbol |
Microarray Raw Intensity Values of MII Oocytes |
|||
|---|---|---|---|---|
|
Average RI† |
IVM/VVM ratio† | P value† | ||
| MII-IVM | MII-VVM | |||
| EGFR | A (1/4) | A (2/4) | ND | NA |
| EGF | 41.68 (3/4) | A (1/4) | Qual | NA |
| AREG | A (1/4) | A (2/4) | ND | NA |
| BTC | A (1/4) | A (0/4) | ND | NA |
| EREG | A (2/4) | A (0/4) | ND | NA |
| TGFα | A (0/4) | A (1/4) | ND | NA |
| CCND1 | 90.20 (3/4) | 65.81 (3/4) | 1.32 | 1.95E-01 |
| CCND2 | 209.19 (4/4) | 197.31 (4/4) | 1.06 | 8.00E-01 |
| CCNE1 | 4,556.88 (4/4) | 4,082.15 (4/4) | 1.12 | 2.41E-01 |
| CCNE2 | A (1/4) | A (0/4) | ND | NA |
| CDK2 | A (0/4) | A (1/4) | ND | NA |
| CDK4 | 248.98 (4/4) | 380.76 (4/4) | 0.65 | 2.92E-01 |
| CDK6 | 56.22 (4/4) | 43.60 (3/4) | 1.29 | 5.65E-01 |
| POLA1 | 631.80 (4/4) | 492.72 (4/4) | 1.28 | 7.14E-04 |
Gene expression microarray data were obtained previously (35). MII, metaphase II of meiosis II. Average raw intensity (RI) values and IVM/VVM (in vitro- to in vivo-matured) ratio and P value calculations were performed for groups that were detected in >3 of 4 replicates. Detection in replicates for each gene is indicated within parentheses. A, group called “absent” when it fails to show detection in >3 of 4 replicates. ND, not applicable due to failure to detect in >3 samples of either group. NA not applicable. Qual, qualitative difference as judged by failure to detect signal in >3 replicates of 1 of the 2 groups.
Table 5.
mRNA expression in rhesus macaque CCs and GCs
| Gene Symbol |
Detection in CCs* |
Detection in GCs*
|
||||||
|---|---|---|---|---|---|---|---|---|
|
No. positive samples |
IVM/VVM† | P value† | No. positive samples
|
IVM/VVM† | P value† | |||
| CC-IVM | CC-VVM | GC-IVM | GC-VVM | |||||
| EGFR | 5/5 | 9/9 | 0.13 | 0.01 | 5/5 | 6/6 | 0.43 | 0.07 |
| EGF | 0/5 | 0/9 | ND | NA | 0/5 | 0/6 | ND | NA |
| AREG | 2/5 | 9/9 | Qual | NA | 5/5 | 6/6 | 6.36E-04 | 2.00E-03 |
| BTC | 5/5 | 9/9 | 0.11 | 0.01 | 5/5 | 6/6 | 0.17 | 0.04 |
| EREG | 4/5 | 9/9 | 0.84 | 0.90 | 5/5 | 6/6 | 0.94 | 0.96 |
| TGFα | 0/5 | 0/9 | ND | NA | 0/5 | 1/6 | ND | NA |
| CCND1 | 4/5 | 9/9 | 0.62 | 0.73 | 5/5 | 6/6 | 0.05 | 0.08 |
| CCND2 | 5/5 | 9/9 | 1.56 | 0.48 | 5/5 | 6/6 | 4.21 | 0.22 |
| CCNE1 | 4/5 | 9/9 | 0.04 | 0.04 | 5/5 | 6/6 | 2.94 | 0.22 |
| CCNE2 | 5/5 | 9/9 | 0.05 | 0.02 | 5/5 | 6/6 | 0.68 | 0.68 |
| CDK2 | 4/5 | 9/9 | 0.17 | 0.15 | 5/5 | 6/6 | 0.39 | 0.34 |
| CDK4 | 4/5 | 9/9 | 2.11 | 0.39 | 5/5 | 6/6 | 0.58 | 0.23 |
| CDK6 | 4/5 | 9/9 | 2.02 | 0.59 | 5/5 | 6/6 | 2.48 | 0.07 |
| POLA1 | 2/5 | 9/9 | Qual | NA | 5/5 | 6/6 | 0.80 | 0.85 |
CC, cumulus cells; GC, granulosa cells.
A sample is called “not detected” when an exponential phase of amplification does not begin before the 40th cycle of qPCR reaction.
IVM/VVM ratio and P value calculations were performed using groups with >3 detections of 5 samples and the samples called “no detection” were excluded from calculations. IVM:VVM <1.0 equals higher levels in vivo, whereas >1.0 equals lower levels in vivo. Qual, qualitative difference as judged by failure to detect signal in >3 replicates of 1 of the cell types. NA, not applicable. ND, not applicable due to no detection in >3 samples of either cell type.
Expression of EGFR pathway genes in rhesus monkey oocytes and cumulus cells.
To explore the EGFR pathway at the molecular level, we examined previously published microarray data on macaque oocytes for the expression of mRNAs encoding EGF, EGF-like ligands, cyclins, and CDKs (Table 4) (35). The EGF mRNA was detected in IVM but not VVM macaque oocytes. The mRNAs encoding the EGF-like ligands AREG, BTC, EREG, and TGFα were not detected in either IVM or VVM macaque oocytes. Raw intensity values for the POLA1 mRNA were higher in IVM oocytes compared with VVM macaque oocytes. The mRNAs encoding cyclins D1, D2, and E1 and CDK4 and CDK6 were present in macaque oocytes, whereas CCNE2 and CDK2 mRNAs were not detected (Table 4).
To explore further the EGFR pathway at the molecular level, we examined the expression of the mRNAs encoding EGF, EGF-like ligands, EGFR, cyclins, and CDKs in the cumulus and granulosa cells by use of a qRT-PCR approach (Table 5). The EGFR mRNA was detected in IVM and VVM cumulus and granulosa cell samples, with expression higher in VVM cumulus cells compared with IVM cumulus cells. The mRNAs encoding the EGF-like factors BTC, AREG, and EREG were detected in IVM and VVM cumulus and granulosa cells, whereas the EGF and TGFα mRNAs were not detected. Cumulus and granulosa cells were also positive for expression of the cyclin D1 (CCND1), cyclin D2 (CCND2), cyclin E1 (CCNE1), cyclin E2 (CCNE2), CDK2, CDK4, CDK6, and POLA1 mRNAs. There was a qualitative difference in both AREG and POLA1 mRNA expression between IVM and VVM cumulus cells, with VVM samples expressing the AREG and POLA1 mRNAs consistently. Expression of the BTC, CCNE1, and CCNE2 mRNAs was significantly higher in VVM cumulus cells compared with IVM cumulus cells. Granulosa cell samples showed higher expression of the AREG and BTC mRNA in VVM samples compared with IVM.
DISCUSSION
Our data reveal for the first time that the EGFR pathway plays an important role in nonhuman primate IVM. Specifically, we observe that inhibition of EGFR signaling significantly inhibits cumulus cell expansion and progression to the metaphase II stage of meiosis and also compromises oocyte quality, as assessed at the level of preimplantaiton development and formation of the inner cell mass. In addition, we find that the EGFR is phosphorylated but that exogenous EGF does not enhance IVM, indicating that endogenous ligands are activating the receptor and that no benefit of additional EGF stimulation can be achieved with exogenous ligand. Last, we report the expression of mRNAs for a number of EGF-like ligands in cumulus and granulosa cells and low or absent expression of these mRNAs in the oocyte, indicating that the expression of the EGF-like factors from the cumulus cells most likely accounts for the ongoing beneficial effects of the EGFR pathway on rhesus monkey IVM.
The presence of EGFR and pEGFR in rhesus macaque COCs is verified here for the first time through immunocytochemistry and supports a role for EGF-like ligands acting directly on cumulus cells through an autocrine/paracrine pathway independent of actions on mural granulosa cells. EGFR has also been found on human cumulus cells and oocytes of various stages of growth and maturation (18, 40, 48, 55, 56). The similar staining of pEGFR in COCs matured in vivo and in vitro indicates that EGFR activation is part of the normal process of oocyte maturation. The EGFR mRNA was detected in some of the macaque oocyte microarray samples, but detection was inconsistent and near background. It should be noted that an “absent” call on an array does not imply zero signal but merely that the signal was too low and/or inconsistent to pass the stringent threshold in use. The confirmation of expression of EGFR in oocytes and pEGFR by immunocytochemistry indicates that the EGFR may be relatively stable, even though the microarray revealed low and inconsistent EGFR mRNA expression. Alternatively, we cannot rule out the possibility that the EGFR antibodies may be binding to an unknown substrate. The presence of EGFR mRNA from qPCR of rhesus macaque cumulus cells confirmed the staining observed. While the expression of EGFR mRNA was higher in cumulus cells matured in vivo compared with in vitro, no quantitative differences in EGFR staining was observed.
The EGFR can be activated by EGF or EGF-like factors, including AREG, EREG, BTC, or TGFα. In mouse ovarian follicles EGFR signaling is essential for normal LH-mediated steroidogenesis (33, 49). In addition, AREG, EREG, and BTC are upregulated in the cumulus cells of mice after injection of hCG in vivo (32). Although the relationship between gonadotropins and EGF-like factors in the rhesus COC has not been investigated, AREG and EREG mRNAs increase in rhesus granulosa cells after an ovulatory stimulus, suggesting involvement during the periovulatory interval (23).
The presence of AG-1478 in IVM culture medium resulted in fewer oocytes matured, decreased cumulus expansion, and a lower percentage of embryos that developed to the blastocyst stage compared with the control group. The lack of significance in the percentage of embryos developing to the blastocyst stage between the vehicle A and AG-1478 treatment groups is likely due to the small sample size, as preliminary studies found no difference between the control and vehicle A groups. The implication of this study that EGF or EGF-like factors have a role in cumulus expansion and other functions in ovarian follicles has also been noted in the mouse (10). This study demonstrates that EGFR activation is important for oocyte maturation in the rhesus macaque, while the addition of exogenous r-hEGF failed to improve already established culture conditions. Therefore, it appears that the presence of cumulus cells could mask the requirement for exogenous r-hEGF, perhaps by secreting EGF-like factors. The oocyte and cumulus cells maintain contact through gap junctions and are in close proximity to each other. Communication through gap junctions and bidirectional paracrine activity between cumulus and oocyte is recognized as critical for improving IVM of oocytes (26, 34).
Few reports exist that examine gene expression profiles in cumulus cells. The PREGER resource, which allows for gene expression analysis of a small cell sample, is critical to providing insight into factors and signaling molecules produced by cumulus cells. Although cumulus cells are required for IVM in nonhuman primates, the structure and function of cumulus cells during the IVM process in rhesus macaques has not been well studied (62, 64). Comparing gene expression profiles of in vitro- and in vivo-matured cumulus cells will help establish what factors are available to the oocyte during maturation and help determine what factors may improve IVM culture conditions.
Expression of EGF mRNA was not detected in rhesus macaque cumulus or granulosa cells but was detected in IVM microarray oocyte samples (35). Studies in the human did not detect EGF mRNA or immunoreactivity in follicles (56, 75). In contrast, a few studies have shown expression of EGF in granulosa cells from various stages of human follicles (40, 59). The lack of EGF mRNA expression from rhesus macaque cumulus and granulosa cells along with the expression of AREG, EREG, and BTC mRNAs in IVM cumulus cells supports our hypothesis that EGF-like factors produced by cumulus cells may be responsible for activation of the EGFR observed during oocyte maturation and mask any effect of exogenous EGF added to the IVM medium. Additionally the presence of EGF mRNA in IVM oocytes may be partly responsible for EGFR activation through an autocrine pathway, although this expression is not mirrored in vivo. The absence of AREG, BTC, EREG, and TGFα mRNA expression from macaque oocytes further supports the importance of the cumulus cells in producing these factors. It is possible that the observed effects may be due to EGFR transactivation however, this is speculative (3).
The presence of AREG, EREG, and BTC mRNAs in rhesus macaque granulosa cells is consistent with a previous report (23). AREG and EREG mRNA are expressed in human granulosa cells, and AREG is expressed in human cumulus cells, both similar to our findings in the rhesus macaque (21, 22). The expression of EGF-like ligands, namely AREG, EREG, and BTC, increases rapidly and transiently after LH/hCG stimulation in mouse ovaries (49). The AREG and EREG mRNAs also increased in expression after LH stimulation in human granulosa cells (22). Higher expression of the AREG and BTC mRNAs in VVM compared with IVM cumulus and granulosa cells in the rhesus macaque is consistent with these reports due to the lack of LH/hCG stimulation in IVM cells. In addition, the decreased expression of AREG and BTC mRNAs from rhesus macaque cumulus cells matured in vitro compared with in vivo demonstrates that culture conditions are suboptimal and do not replace in vivo conditions.
The addition of r-hEGF to the IVM culture medium did not affect oocyte nuclear maturation, COC cytoskeletal elements, or development of subsequent embryos in the rhesus macaque. This contrasts with the increased maturation rates observed in other species when EGF or EGF-like factors were added to the culture medium: murine (16), bovine (13, 37, 38), rabbit (39), sheep (30), rat (15), and pig (11). For the rhesus monkey, successful IVM has only been achieved with cumulus-enclosed oocytes, indicating that it is crucial that COCs remain intact during IVM (65). In the human, EGF in IVM culture medium improves maturation rates from naked germinal vesicle stage oocytes but has no effect on COCs (29). This is consistent with our results in demonstrating that the presence of r-hEGF does not improve nuclear maturation for cumulus-enclosed oocytes. Another study in the human found EGF to improve maturation of oocytes from nonstimulated ovaries, making comparsions with macaque oocytes from FSH-stimulated ovaries difficult (28). Differences exist in the remodeling of cumulus cell transzonal processes and the cytoplasmic microtubule network in IVM oocytes compared with VVM oocytes in the rhesus macaque, suggesting that culture conditions continue to be suboptimal (46). The addition of exogenous r-hEGF did not affect the number of actin and tubulin transzonal processes, number of gap junctions, or cytoplasmic microtubule scores suggesting that the addition of r-hEGF did not improve conditions comparable to those in vivo.
Any action of EGF on the COC may be mediated by changes in expression of cell cycle-related genes, namely cyclins and cyclin-dependent kinases (CDKs). Granulosa cells cease proliferation when stimulated with LH (9, 57, 60) and exit the cell cycle within 12 h of the LH surge in primates (9). In addition, changes in expression of G1/S phase transition cyclins and CDKs accompany granulosa cell proliferation (7). Interestingly, there are data suggesting that, in marked contrast to mural granulosa cells, cumulus cells continue to transit the cell cycle following an ovulatory stimulus (6, 32). Thus, changes in cell cycle dynamics in cumulus cells are not as clear and warrant further investigation. One possible role of EGF at the cumulus may be to promote G1/S phase transit, thereby causing cumulus expansion. The presence of the following cyclins and CDKs in cumulus and granulosa cells only begins to develop this hypothesis by providing evidence of their expression. CCND/CDK4 and CDK6 are important for the early transition through G1 of the cell cycle, whereas CCNE/CDK2 is involved in the progression through the G1/S phase of the cell cycle. A previous study found no change in the expression of CCND2 or CCNE mRNA in rhesus macaque granulosa cells following an ovulatory stimulus, similar to our results (9). In contrast, the expression pattern of CCND2 and CCNE mRNA in rhesus macaque cumulus cells after IVM and VVM is reported here for the first time. CCNE1 and CCNE2 mRNA expression increased in VVM compared with IVM cumulus cells, whereas CCND1 and CCND2 mRNA expression was unchanged. The importance of the CCNE/CDK2 complex in normal granulosa or cumulus cell proliferation is unknown, and the potential for reduced activity due to posttranslational changes should not be ruled out. The decreased expression of CCNE1 and CCNE2 mRNA expression from in vitro-cultured cumulus cells further demonstrates that IVM COCs are different from VVM COCs at the molecular level. These differences in gene expression may influence IVM oocyte quality.
This report presents a direct comparison of gene expression in nonhuman primate cumulus and granulosa cells. The analysis of gene expression from small samples of cumulus and granulosa cells in the nonhuman primate has been made possible through PREGER and a novel RT-PCR approach (58). This resource allows us to obtain a molecular sketch of what factors may be available to the oocyte and influence oocyte maturation. This information is essential when designing and optimizing a culture system. The addition of individual growth factors to IVM culture media has proven an inefficient strategy for enhancing IVM in the rhesus monkey compared with other species. In light of this study, the characterization of factors secreted by cumulus cells during maturation in vivo and in vitro is necessary to circumvent the addition of growth factors already present and to clarify the factors that might be effective in optimization of culture media.
GRANTS
This research was supported by National Institutes of Health grants RR-13439 (C. A. VandeVoort), RR-00169 (California National Primate Research Center), HD-043358 (C. L. Chaffin), and RR-15253 (K. E. Latham).
Acknowledgments
We thank Dana Hill for technical assistance, microscopy work, and image preparation and Sarah Rodenburg for measuring cumulus expansion.
REFERENCES
- 1.Allworth AE, Albertini DF. Meiotic maturation in cultured bovine oocytes is accompanied by remodeling of the cumulus cell cytoskeleton. Devel Biol 158: 101–112, 1993. [DOI] [PubMed] [Google Scholar]
- 2.Bavister BD, Boatman DE, Leibfried L, Loose M, Vernon MW. Fertilization and cleavage of rhesus monkey oocytes in vitro. Biol Reprod 28: 983–999, 1983. [DOI] [PubMed] [Google Scholar]
- 3.Bhola NE, Grandis JR. Crosstalk between G-protein-coupled receptors and epidermal growth factor receptor in cancer. Front Biosci 13: 1857–1865, 2008. [DOI] [PubMed] [Google Scholar]
- 4.Boatman DE In vitro growth of non-human primate pre- and peri- implantation embryos. In: The Mammalian Preimplantation Embryo: Regulation of Growth and Differentiation In Vitro, edited by Bavister BD. New York: Plenum, 1987, p. 273–308.
- 5.Brower PT, Schultz RM. Intercellular communication between granulosa cells and mouse oocytes: existence and possible nutritional role during oocyte growth. Devel Biol 90: 144–153, 1982. [DOI] [PubMed] [Google Scholar]
- 6.Cannon JD, Cherian-Shaw M, Chaffin CL. Proliferation of rat granulosa cells during the periovulatory interval. Endocrinology 146: 414–422, 2005. [DOI] [PubMed] [Google Scholar]
- 7.Cannon JD, Cherian-Shaw M, Lovekamp-Swan T, Chaffin CL. Granulosa cell expression of G1/S phase cyclins and cyclin-dependent kinases in PMSG-induced follicle growth. Mol Cell Endocrinol 264: 6–15, 2007. [DOI] [PubMed] [Google Scholar]
- 8.Cha KY, Chian RC. Maturation in vitro of immature human oocytes for clinical use. Hum Reprod Update 4: 103–120, 1998. [DOI] [PubMed] [Google Scholar]
- 9.Chaffin CL, Schwinof KM, Stouffer RL. Gonadotropin and steroid control of granulosa cell proliferation during the periovulatory interval in rhesus monkeys. Biol Reprod 65: 755–762, 2001. [DOI] [PubMed] [Google Scholar]
- 10.Conti M, Hsieh M, Park JY, Su YQ. Role of the epidermal growth factor network in ovarian follicles. Mol Endocrinol 20: 715–723, 2006. [DOI] [PubMed] [Google Scholar]
- 11.Coskun S, Lin YC. Effects of transforming growth factors and activin-A on in vitro porcine oocyte maturation. Mol Reprod Dev 38: 153–159, 1994. [DOI] [PubMed] [Google Scholar]
- 12.Coskun S, Lin YC. Mechanism of action of epidermal growth factor-induced porcine oocyte maturation. Mol Reprod Dev 42: 311–317, 1995. [DOI] [PubMed] [Google Scholar]
- 13.Coskun S, Sanbuissho A, Lin YC, Rikihisa Y. Fertilizability and subsequent developmental ability of bovine oocytes matured in medium containing epidermal growth factor (EGF). Theriogenology 36: 485–494, 1991. [DOI] [PubMed] [Google Scholar]
- 14.De La Fuente R, O'Brien MJ, Eppig JJ. Epidermal growth factor enhances preimplantation developmental competence of maturing mouse oocytes. Hum Reprod 14: 3060–3068, 1999. [DOI] [PubMed] [Google Scholar]
- 15.Dekel N, Sherizly I. Epidermal growth factor induces maturation of rat follicle-enclosed oocytes. Endocrinology 116: 406–409, 1985. [DOI] [PubMed] [Google Scholar]
- 16.Downs SM Specificity of epidermal growth factor action on maturation of the murine oocyte and cumulus oophorus in vitro. Biol Reprod 41: 371–379, 1989. [DOI] [PubMed] [Google Scholar]
- 17.Edwards RG Maturation in vitro of mouse, sheep, cow, pig, rhesus monkey and human ovarian oocytes. Nature 208: 349–351, 1965. [DOI] [PubMed] [Google Scholar]
- 18.el-Danasouri I, Frances A, Westphal LM. Immunocytochemical localization of transforming growth factor-alpha and epidermal growth factor receptor in human fallopian tubes and cumulus cells. Am J Reprod Immunol 30: 82–87, 1993. [DOI] [PubMed] [Google Scholar]
- 19.Eppig JJ A comparison between oocyte growth in coculture with granulosa cells and oocytes with granulosa cell-oocyte junctional contact maintained in vitro. J Exper Zool 209: 345–353, 1979. [DOI] [PubMed] [Google Scholar]
- 20.Eppig JJ Oocyte control of ovarian follicular development and function in mammals. Reproduction (Cambridge) 122: 829–838, 2001. [DOI] [PubMed] [Google Scholar]
- 21.Feuerstein P, Cadoret V, Dalbies-Tran R, Guerif F, Bidault R, Royere D. Gene expression in human cumulus cells: one approach to oocyte competence. Hum Reprod 22: 3069–3077, 2007. [DOI] [PubMed] [Google Scholar]
- 22.Freimann S, Ben-Ami I, Dantes A, Ron-El R, Amsterdam A. EGF-like factor epiregulin and amphiregulin expression is regulated by gonadotropins/cAMP in human ovarian follicular cells. Biochem Biophys Res Commun 324: 829–834, 2004. [DOI] [PubMed] [Google Scholar]
- 23.Fru KN, Cherian-Shaw M, Puttabyatappa M, VandeVoort CA, Chaffin CL. Regulation of granulosa cell proliferation and EGF-like ligands during the periovulatory interval in monkeys. Hum Reprod 22: 1247–1252, 2007. [DOI] [PubMed] [Google Scholar]
- 24.Galli C, Moor RM. Gonadotrophin requirements for the in vitro maturation of sheep oocytes and their subsequent embryonic development. Theriogenology 35: 1083–1093, 1991. [Google Scholar]
- 25.Gilchrist RB, Lane M, Thompson JG. Oocyte-secreted factors: regulators of cumulus cell function and oocyte quality. Hum Reprod Update 14: 159–177, 2008. [DOI] [PubMed] [Google Scholar]
- 26.Gilchrist RB, Thompson JG. Oocyte maturation: emerging concepts and technologies to improve developmental potential in vitro. Theriogenology 67: 6–15, 2007. [DOI] [PubMed] [Google Scholar]
- 27.Gomez E, de los Santos MJ, Ruiz A, Tarin JJ, Remohi J, Pellicer A. Effects of epidermal growth factor in the final stages of nuclear and cytoplasmic oocyte maturation in humans. Hum Reprod 8: 691–694, 1993. [DOI] [PubMed] [Google Scholar]
- 28.Gomez E, Tarin JJ, Pellicer A. Oocyte maturation in humans: the role of gonadotropins and growth factors. Fertil Steril 60: 40–46, 1993. [PubMed] [Google Scholar]
- 29.Goud PT, Goud AP, Qian C, Laverge H, Van der Elst J, De Sutter P, Dhont M. In-vitro maturation of human germinal vesicle stage oocytes: role of cumulus cells and epidermal growth factor in the culture medium. Hum Reprod 13: 1638–1644, 1998. [DOI] [PubMed] [Google Scholar]
- 30.Guler A, Poulin N, Mermillod P, Terqui M, Cognie Y. Effect of growth factors, EGF and IGF-I, and estradiol on in vitro maturation of sheep oocytes. Theriogenology 54: 209–218, 2000. [DOI] [PubMed] [Google Scholar]
- 31.Harper KM, Brackett BG. Bovine blastocyst development after in vitro maturation in a defined medium with epidermal growth factor and low concentrations of gonadotropins. Biol Reprod 48: 409–416, 1993. [DOI] [PubMed] [Google Scholar]
- 32.Hernandez-Gonzalez I, Gonzalez-Robayna I, Shimada M, Wayne CM, Ochsner SA, White L, Richards JS. Gene expression profiles of cumulus cell oocyte complexes during ovulation reveal cumulus cells express neuronal and immune-related genes: does this expand their role in the ovulation process? Mol Endocrinol 20: 1300–1321, 2006. [DOI] [PubMed] [Google Scholar]
- 33.Jamnongjit M, Gill A, Hammes SR. Epidermal growth factor receptor signaling is required for normal ovarian steroidogenesis and oocyte maturation. Proc Natl Acad Sci USA 102: 16257–16262, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kimura N, Hoshino Y, Totsukawa K, Sato E. Cellular and molecular events during oocyte maturation in mammals: molecules of cumulus-oocyte complex matrix and signalling pathways regulating meiotic progression. Soc Reprod Fertil Suppl 63: 327–342, 2007. [PubMed] [Google Scholar]
- 35.Lee YS, Latham KE, VandeVoort CA. Effects of in vitro maturation on gene expression in rhesus monkey oocytes. Physiol Genomics 35: 145–158, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta CT) method. Methods 25: 402–408, 2001. [DOI] [PubMed] [Google Scholar]
- 37.Lonergan P, Carolan C, Van Langendonckt A, Donnay I, Khatir H, Mermillod P. Role of epidermal growth factor in bovine oocyte maturation and preimplantation embryo development in vitro. Biol Reprod 54: 1420–1429, 1996. [DOI] [PubMed] [Google Scholar]
- 38.Lorenzo PL, Illera MJ, Illera JC, Illera M. Enhancement of cumulus expansion and nuclear maturation during bovine oocyte maturation in vitro by the addition of epidermal growth factor and insulin-like growth factor I. J Reprod Fertil 101: 697–701, 1994. [DOI] [PubMed] [Google Scholar]
- 39.Lorenzo PL, Rebollar PG, Illera MJ, Illera JC, Illera M, Alvarino JM. Stimulatory effect of insulin-like growth factor I and epidermal growth factor on the maturation of rabbit oocytes in vitro. J Reprod Fertil 107: 109–117, 1996. [DOI] [PubMed] [Google Scholar]
- 40.Maruo T, Ladines-Llave CA, Samoto T, Matsuo H, Manalo AS, Ito H, Mochizuki M. Expression of epidermal growth factor and its receptor in the human ovary during follicular growth and regression. Endocrinology 132: 924–931, 1993. [DOI] [PubMed] [Google Scholar]
- 41.Matzuk MM, Burns KH, Viveiros MM, Eppig JJ. Intercellular communication in the mammalian ovary: oocytes carry the conversation. Science 296: 2178–2180, 2002. [DOI] [PubMed] [Google Scholar]
- 42.McKiernan SH, Bavister BD. Culture of one-cell hamster embryos with water soluble vitamins: pantothenate stimulates blastocyst production. Hum Reprod 15: 157–164, 2000. [DOI] [PubMed] [Google Scholar]
- 43.Mermillod P, Oussaid B, Cognie Y. Aspects of follicular and oocyte maturation that affect the developmental potential of embryos. J Reprod Fertil Suppl 54: 449–460, 1999. [PubMed] [Google Scholar]
- 44.Morgan PM, Warikoo PK, Bavister BD. In vitro maturation of ovarian oocytes from unstimulated rhesus monkeys: assessment of cytoplasmic maturity by embryonic development after in vitro fertilization. Biol Reprod 45: 89–93, 1991. [DOI] [PubMed] [Google Scholar]
- 45.Mtango NR, Latham KE. Differential expression of cell cycle genes in rhesus monkey oocytes and embryos of different developmental potentials. Biol Reprod 78: 254–266, 2008. [DOI] [PubMed] [Google Scholar]
- 46.Nyholt de Prada JK, Hill DL, Chaffin CL, VandeVoort CA. Nuclear maturation and structural components of nonhuman primate cumulus-oocyte complexes during in vivo and in vitro maturation. Fertil Steril (In Press): 2008. [DOI] [PMC free article] [PubMed]
- 47.Nyholt de Prada JK, VandeVoort CA. Growth hormone and in vitro maturation of rhesus macaque oocytes and subsequent embryo development. J Assisted Reprod Genetics 25: 145–158, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ozdogan B, Erdogan D, Take G, Ozogul C. Immunohistochemical localization of epidermal growth factor receptor (EGF-r) and transforming growth factor alpha (TGF-alpha) in developing human ovarian follicles. Acta Physiologica Hungarica 92: 53–66, 2005. [DOI] [PubMed] [Google Scholar]
- 49.Park JY, Su YQ, Ariga M, Law E, Jin SL, Conti M. EGF-like growth factors as mediators of LH action in the ovulatory follicle. Science 303: 682–684, 2004. [DOI] [PubMed] [Google Scholar]
- 50.Pfaffl MW, Horgan GW, Dempfle L. Relative expression software tool (REST) for group-wise comparison and statistical analysis of relative expression results in real-time PCR. Nucleic Acids Res 30: e36, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Picton HM Oocyte maturation in vitro. Curr Opin Obstet Gynecol 14: 295–302, 2002. [DOI] [PubMed] [Google Scholar]
- 52.Pinyopummintr T, Bavister BD. Effects of amino acids on development in vitro of cleavage-stage bovine embryos into blastocysts. Reprod Fertil Devel 8: 835–841, 1996. [DOI] [PubMed] [Google Scholar]
- 53.Pinyopummintr T, Bavister BD. Energy substrate requirements for in vitro development of early cleavage-stage bovine embryos. Mol Reprod Dev 44: 193–199, 1996. [DOI] [PubMed] [Google Scholar]
- 54.Prochazka R, Kalab P, Nagyova E. Epidermal growth factor-receptor tyrosine kinase activity regulates expansion of porcine oocyte-cumulus cell complexes in vitro. Biol Reprod 68: 797–803, 2003. [DOI] [PubMed] [Google Scholar]
- 55.Qu J, Godin PA, Nisolle M, Donnez J. Distribution and epidermal growth factor receptor expression of primordial follicles in human ovarian tissue before and after cryopreservation. Hum Reprod 15: 302–310, 2000. [DOI] [PubMed] [Google Scholar]
- 56.Qu J, Nisolle M, Donnez J. Expression of transforming growth factor-alpha, epidermal growth factor, and epidermal growth factor receptor in follicles of human ovarian tissue before and after cryopreservation. Fertil Steril 74: 113–121, 2000. [DOI] [PubMed] [Google Scholar]
- 57.Quirk SM, Cowan RG, Harman RM. Progesterone receptor and the cell cycle modulate apoptosis in granulosa cells. Endocrinology 145: 5033–5043, 2004. [DOI] [PubMed] [Google Scholar]
- 58.Rambhatla L, Patel B, Dhanasekaran N, Latham KE. Analysis of G protein alpha subunit mRNA abundance in preimplantation mouse embryos using a rapid, quantitative RT-PCR approach. Mol Reprod Dev 41: 314–324, 1995. [DOI] [PubMed] [Google Scholar]
- 59.Reeka N, Berg FD, Brucker C. Presence of transforming growth factor alpha and epidermal growth factor in human ovarian tissue and follicular fluid. Hum Reprod 13: 2199–2205, 1998. [DOI] [PubMed] [Google Scholar]
- 60.Robker RL, Richards JS. Hormone-induced proliferation and differentiation of granulosa cells: a coordinated balance of the cell cycle regulators cyclin D2 and p27Kip1. Mol Endocrinol 12: 924–940, 1998. [DOI] [PubMed] [Google Scholar]
- 61.Sarason RL, VandeVoort CA, Mader DR, Overstreet JW. The use of nonmetal electrodes in electroejaculation of restrained but unanesthetized macaques. J Med Primatol 20: 122–125, 1991. [PubMed] [Google Scholar]
- 62.Schramm RD, Bavister BD. Effects of granulosa cells and gonadotrophins on meiotic and developmental competence of oocytes in vitro in non-stimulated rhesus monkeys. Hum Reprod 10: 887–895, 1995. [DOI] [PubMed] [Google Scholar]
- 63.Schramm RD, Bavister BD. Follicle-stimulating hormone priming of rhesus monkeys enhances meiotic and developmental competence of oocytes matured in vitro. Biol Reprod 51: 904–912, 1994. [DOI] [PubMed] [Google Scholar]
- 64.Schramm RD, Bavister BD. Granulosa cells from follicle stimulating hormone-primed monkeys enhance the development competence of in-vitro-matured oocytes from non-stimulated rhesus monkeys. Hum Reprod 11: 1698–1702, 1996. [DOI] [PubMed] [Google Scholar]
- 65.Schramm RD, Bavister BD. A macaque model for studying mechanisms controlling oocyte development and maturation in human and non-human primates. Hum Reprod 14: 2544–2555, 1999. [DOI] [PubMed] [Google Scholar]
- 66.Schramm RD, Paprocki AM. Birth of rhesus monkey infant after transfer of embryos derived from in-vitro matured oocytes: short communication. Hum Reprod 15: 2411–2414, 2000. [DOI] [PubMed] [Google Scholar]
- 67.Schramm RD, Paprocki AM, VandeVoort CA. Causes of developmental failure of in-vitro matured rhesus monkey oocytes: impairments in embryonic genome activation. Hum Reprod 18: 826–833, 2003. [DOI] [PubMed] [Google Scholar]
- 68.Schramm RD, Tennier MT, Boatman DE, Bavister BD. Chromatin configurations and meiotic competence of oocytes are related to follicular diameter in nonstimulated rhesus monkeys. Biol Reprod 48: 349–356, 1993. [DOI] [PubMed] [Google Scholar]
- 69.Schroeder AC, Eppig JJ. The developmental capacity of mouse oocytes that matured spontaneously in vitro is normal. Devel Biol 102: 493–497, 1984. [DOI] [PubMed] [Google Scholar]
- 70.Shimada M, Hernandez-Gonzalez I, Gonzalez-Robayna I, Richards JS. Paracrine and autocrine regulation of epidermal growth factor-like factors in cumulus oocyte complexes and granulosa cells: key roles for prostaglandin synthase 2 and progesterone receptor. Mol Endocrinol 20: 1352–1365, 2006. [DOI] [PubMed] [Google Scholar]
- 71.Sibilia M, Kroismayr R, Lichtenberger BM, Natarajan A, Hecking M, Holcmann M. The epidermal growth factor receptor: from development to tumorigenesis. Differentiation Res Biol Diversity 75: 770–787, 2007. [DOI] [PubMed] [Google Scholar]
- 72.Sirard MA, Parrish JJ, Ware CB, Leibfried-Rutledge ML, First NL. The culture of bovine oocytes to obtain developmentally competent embryos. Biol Reprod 39: 546–552, 1988. [DOI] [PubMed] [Google Scholar]
- 73.Stewart DR, Vandevoort CA. Simulation of human luteal endocrine function with granulosa lutein cell culture. J Clin Endocrinol Metab 82: 3078–3083, 1997. [DOI] [PubMed] [Google Scholar]
- 74.Su YQ, Wu X, O'Brien MJ, Pendola FL, Denegre JN, Matzuk MM, Eppig JJ. Synergistic roles of BMP15 and GDF9 in the development and function of the oocyte-cumulus cell complex in mice: genetic evidence for an oocyte-granulosa cell regulatory loop. Devel Biol 276: 64–73, 2004. [DOI] [PubMed] [Google Scholar]
- 75.Tamura M, Sasano H, Suzuki T, Fukaya T, Funayama Y, Takayama K, Takaya R, Yajima A. Expression of epidermal growth factors and epidermal growth factor receptor in normal cycling human ovaries. Hum Reprod 10: 1891–1896, 1995. [DOI] [PubMed] [Google Scholar]
- 76.Van den Hurk R, Abir R, Telfer EE, Bevers MM. Primate and bovine immature oocytes and follicles as sources of fertilizable oocytes. Hum Reprod Update 6: 457–474, 2000. [DOI] [PubMed] [Google Scholar]
- 77.VandeVoort CA, Baughman WL, Stouffer RL. Comparison of different regimens of human gonadotropins for superovulation of rhesus monkeys: ovulatory response and subsequent luteal function. J In Vitro Fert Embryo Transf 6: 85–91, 1989. [DOI] [PubMed] [Google Scholar]
- 78.VandeVoort CA, Leibo SP, Tarantal AF. Improved collection and developmental competence of immature macaque oocytes. Theriogenology 59: 699–707, 2003. [DOI] [PubMed] [Google Scholar]
- 79.Wolf DP, Vandevoort CA, Meyer-Haas GR, Zelinski-Wooten MB, Hess DL, Baughman WL, Stouffer RL. In vitro fertilization and embryo transfer in the rhesus monkey. Biol Reprod 41: 335–346, 1989. [DOI] [PubMed] [Google Scholar]
- 80.Zheng P, Bavister BD, Ji WZ. Amino acid requirements for maturation of rhesus monkey (Macacca mulatta) oocytes in culture. Reproduction (Cambridge) 124: 515–522, 2002. [DOI] [PubMed] [Google Scholar]
- 81.Zheng P, Patel B, McMenamin M, Moran E, Paprocki AM, Kihara M, Schramm RD, Latham KE. Effects of follicle size and oocyte maturation conditions on maternal messenger RNA regulation and gene expression in rhesus monkey oocytes and embryos. Biol Reprod 72: 890–897, 2005. [DOI] [PubMed] [Google Scholar]
- 82.Zheng P, Patel B, McMenamin M, Paprocki AM, Schramm RD, Nagl NG Jr, Wilsker D, Wang X, Moran E, Latham KE. Expression of genes encoding chromatin regulatory factors in developing rhesus monkey oocytes and preimplantation stage embryos: possible roles in genome activation. Biol Reprod 70: 1419–1427, 2004. [DOI] [PubMed] [Google Scholar]
- 83.Zheng P, Patel B, McMenamin M, Reddy SE, Paprocki AM, Schramm RD, Latham KE. The primate embryo gene expression resource: a novel resource to facilitate rapid analysis of gene expression patterns in non-human primate oocytes and preimplantation stage embryos. Biol Reprod 70: 1411–1418, 2004. [DOI] [PubMed] [Google Scholar]
- 84.Zheng P, Si W, Wang H, Zou R, Bavister BD, Ji W. Effect of age and breeding season on the developmental capacity of oocytes from unstimulated and follicle-stimulating hormone-stimulated rhesus monkeys. Biol Reprod 64: 1417–1421, 2001. [DOI] [PubMed] [Google Scholar]
- 85.Zheng P, Wang H, Bavister BD, Ji W. Maturation of rhesus monkey oocytes in chemically defined culture media and their functional assessment by IVF and embryo development. Hum Reprod 16: 300–305, 2001. [DOI] [PubMed] [Google Scholar]