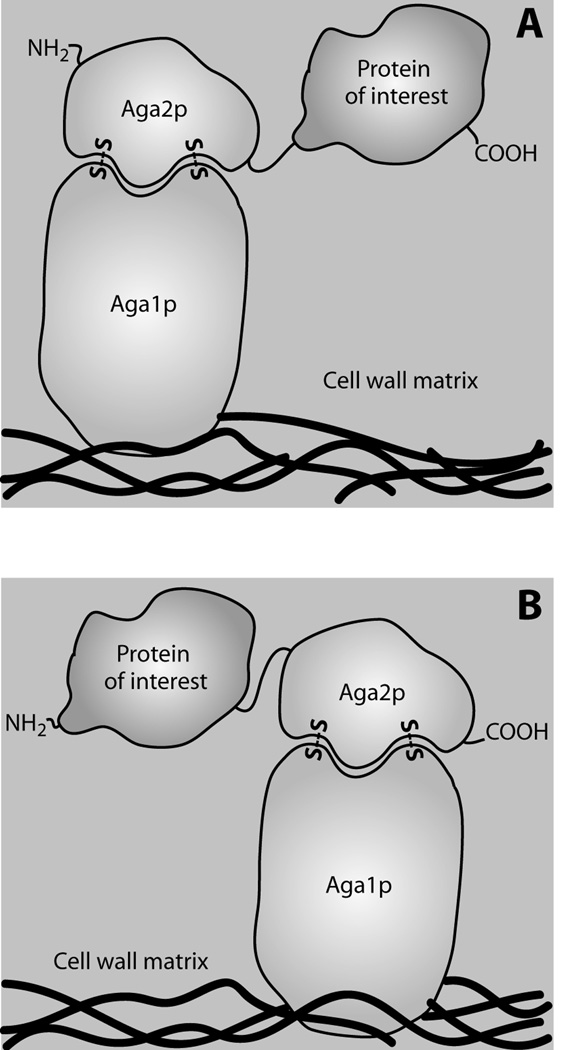
Figure 1.
Mode of Aga2p linkage may affect expression and function of yeast surface displayed target proteins. (A) Expression construct that fuses the protein of interest by its N-terminus to the C-terminus of Aga2p, via a flexible linker. Extracellular secretion is thus directed by the native Aga2p signal peptide. (B) Construct that fuses the protein of interest by its C-terminus to the N-terminus of Aga2p. A signal peptide must therefore be included at the N-terminus of this construct to direct secretion. In both construct formats, Aga2p is bound to the Aga1p subunit, which is in turn covalently linked to cell wall glucans83. A similar cell wall linkage is used by most of the alternate anchoring fusions discussed herein. In both constructs, the protein of interest is typically flanked on its N- and C-terminus by epitope tags. The protein of interest can be oligomeric, in which case one subunit is expressed as an Aga2p fusion while others require a signal peptide to direct them to the secretory pathway for assembly.