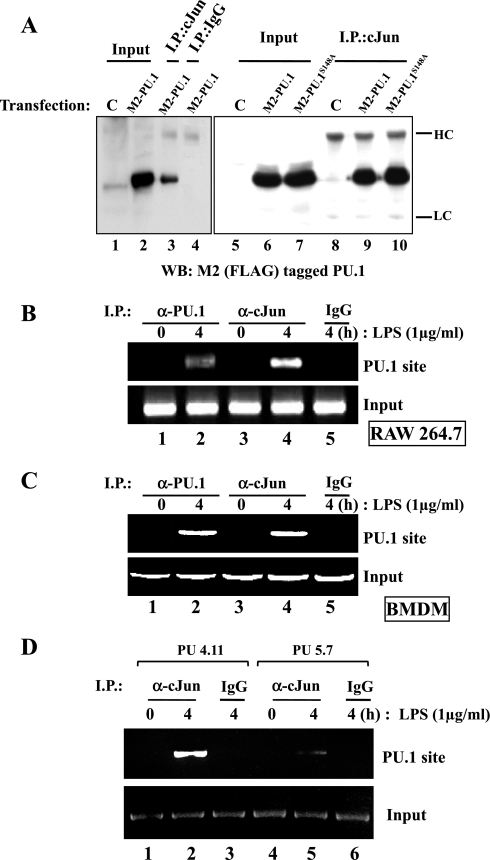
Fig. 4.
PU.1 interacts with cJun in the PU.1 binding site of the L-PGDS promoter. A: HEK-293 cells were transfected with pCMV (C) or a plasmid encoding FLAG-tagged PU.1 or PU.1S148A. Total cell lysate was incubated with an α-cJun antibody (lanes 3, 8, 9, and 10) or with an isotypic IgG (lane 4) to exclude nonspecific precipitation. The immune complexes precipitated with the antibodies were analyzed by Western blotting with M2 antibody. One-tenth of total cell lysate was used as input controls (lanes 1, 2, 5, 6, and 7). HC and LC represent heavy and light chains of an immunoglobulin, respectively. A ChIP assay was performed with RAW 264.7 cells (B) or BMDM of C57BL/6 mice (C) treated with LPS for 4 h. The nuclear fractions were equally divided and added with either an α-PU.1 antibody (lanes 1 and 2, top) or an α-cJun antibody (lanes 3 and 4). DNA eluted from each precipitated immune complex was amplified by PCR with the set of primers flanking the PU.1 binding site of the L-PGDS promoter. DNA precipitated by an isotypic IgG (lane 5) and 100 ng of genomic DNA were used as controls (bottom). D: similarly, we performed a ChIP assay with PU5.7, along with the control, PU4.11, after treatment with LPS for 4 h. The nuclear fractions were added with the α-cJun antibody (lanes 1, 2, 4, and 5) or the isotypic IgG (lane 3 and 6) to exclude nonspecific precipitation. Eluted DNA was analyzed by PCR as described above.