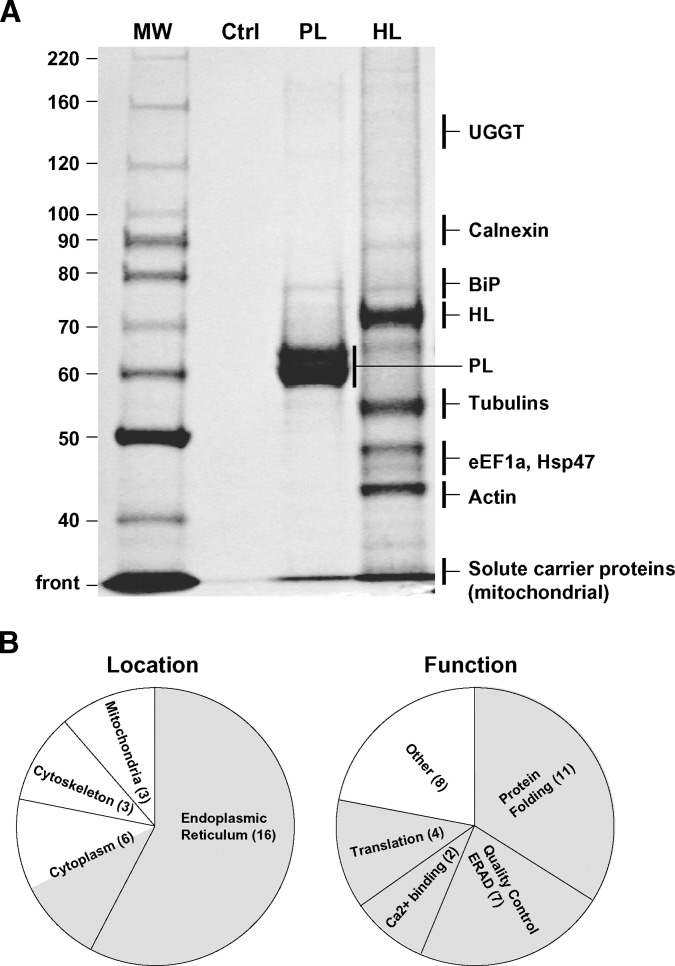
Fig. 2.
A: Representative SDS PAGE gel containing silver-stained proteins isolated by TAP using PL or HL as entry points. The control (Ctrl) lane is a TAP of CHO lysates that have not been transfected with any TAP constructs. Shown on the right are regions of the gel containing some examples of proteins copurifying with an HL-TAP construct (see Tables 1, 2 for a comprehensive list). Only BiP, mitochondrial solute carrier proteins (PTP and ANT2), and GAPDH copurified with the PL-TAP construct. B: All proteins copurifying with HL in all experiments have been combined and presented with regard to location or function. The number of proteins identified in a given class is given in parentheses. The shaded areas represent locations and functions consistent with proteins that may directly or indirectly interact with HL during its co and posttranslational maturation and degradation.