Fig. 6.
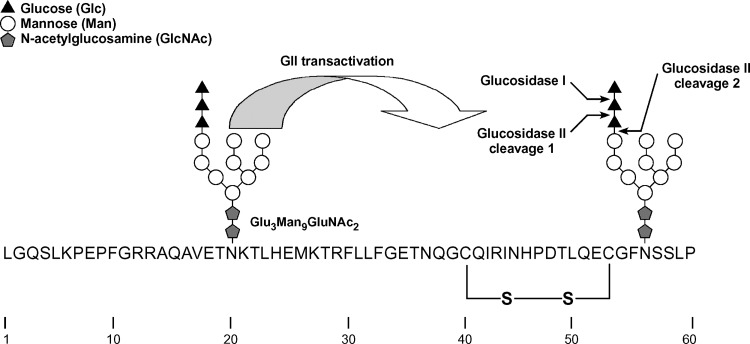
The sequence and glycosylation of the first N-terminal 60 amino acid residues of human HL lacking the signal peptide. Two N-linked glycosylation sites are present at positions 20 and 56. The glycan structure shown is the unprocessed Glc3Man9GlcNAc2 that is transferred by the oligosaccharyl transferase to the growing polypeptide chain. The first two processing events are removal of the outermost glucose residue by glucosidase I (GI), followed by removal of the second glucose residue by GII. The removal of the second glucose residue from the N-linked glycan at Asn56 is greatly facilitated by transactivation of GII by the N-linked glycan at Asn20 (see text). The resulting Glc1Man9GlcNAc2 is a substrate for CNX binding. The removal of the final glucose residue is also catalyzed by GII, and its removal signals the release of HL from CNX. This second cleavage is not affected by transactivation. Notice the close juxtaposition of the first disulfide bond in HL (Cys40—Cys53) with regard to the second N-glycan chain at Asn56.