Figure 1.
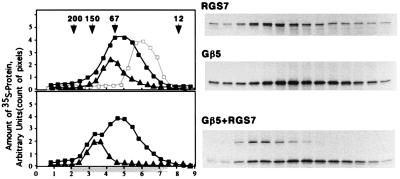
Gβ5-RGS7 interaction in vitro. (Upper) Overlay plot of three experiments resolving monomeric Gβ5 (■), Gβ5 with excess Gγ2 (□, gray line), and monomeric RGS7 (▴) on a Superdex 200 gel-filtration column as described in Materials and Methods. The G protein γ subunit Gγ2 was synthesized in the presence of nonradioactive methionine. The Gβ5γ2 complex has a lower apparent molecular weight than Gβ5 apparently because of a more compact structure (20). (Lower) Experiment with the mixture of Gβ5 with RGS7 (squares, position of Gβ5; triangles, RGS7). x axis: Elution volume (ml), starting (zero) at the beginning of elution of the blue dextran. Highlighted area below the axis denotes the fractions resolved by SDS/PAGE and radioautography, shown to the right. y axis: Arbitrary units based on the strength of the bands on the gel determined by the amount of pixels per band. The fractions were analyzed by SDS/PAGE followed by radioautography, and the amount of 35S-labeled Gβ5 or RGS7 was measured by image analysis of the exposed film using the nih image software. Each experiment was done at least two times, each with an independent in vitro translation.