Abstract
Men exhibit a higher incidence of cardiovascular diseases than do women. The cardiovascular actions of sex steroids have been suggested as primary factors in mediating this sex difference. The mechanisms by which sex steroids, androgens and estrogens, mediate cardiovascular actions remain unclear. Excess aldosterone secretion has been associated with cardiovascular diseases. The hypothesis tested in this study was that at physiological concentrations, androgens stimulate and estradiol inhibits aldosterone secretion by human adrenal cells. In contrast to our hypothesis, physiological concentrations of sex steroids did not modify aldosterone secretion by H295R human adrenocortical cells. However, supraphysiological concentrations (300–1000 nM) of dihydrotestosterone (DHT) significantly stimulated basal and Angiotensin II-mediated aldosterone secretion. The stimulatory effect of DHT on aldosterone secretion was not blocked by the classical androgen receptor blocker flutamide. The stimulatory effect of DHT on aldosterone secretion was also independent of the intra-adrenal renin angiotensin system since it was neither modified by treatment with the Angiotensin II receptor type 1 blocker losartan or the Angiotensin Converting Enzyme inhibitor captopril. Inhibitors of the calmodulin/calmodulin-dependent protein kinase (CaMK) and protein kinase C intracellular signaling pathways abolished the DHT stimulatory effect on aldosterone secretion by H295R cells. In conclusion, physiological concentrations of sex steroids did not modify aldosterone secretion by human adrenal cells. However, supraphysiological concentrations of DHT stimulated aldosterone secretion by human adrenal cells by the calmodulin/CaMK and protein kinase C intracellular signaling pathways but independently of the classical androgen receptor. Supraphysiological doses of androgen may promote cardiovascular diseases via stimulation of aldosterone secretion.
Keywords: adrenal cells, androgens, dihydrotestosterone, estradiol, aldosterone
INTRODUCTION
Male sex is an independent risk factor for cardiovascular diseases (Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults, (2001). Men also progress faster to end stage renal disease than women (Neugarten et al. 2000). However, the mechanisms responsible for the gender difference observed in cardiovascular and renal diseases remain unknown. Several studies suggest that sex hormones, testosterone and estradiol, are important factors mediating the gender difference in cardiovascular diseases (Mendelsohn et al. 1999 Mendelsohn et al. 2001; Baker et al. 2003; Liu et al. 2003; Muller et al. 2003; Wu et al. 2003; Phillips 2005). For example, testosterone mediates hypertension and renal injury in several animal models (Reckelhoff et al. 2000; Ji et al. 2005; Song et al. 2006; Yanes et al. 2008). On the other hand, estradiol has been shown to have many actions on cardiovascular system that are mainly protective (Dean et al. 2005).
Aldosterone, the main mineralocorticoid produced by the zona glomerulosa of the adrenal gland, plays a major role in water and electrolyte homeostasis. Excess aldosterone, on the other hand, causes hypertension and target organ damage (Rocha et al. 2001; Struthers 2004). Several lines of evidence have suggested a relation between sex steroids and aldosterone. In humans, under strict controlled salt intake conditions, men have significantly higher circulating aldosterone levels compared with age-matched women (Miller et al. 1999). Also, aldosterone levels are higher in male New Zealand genetically hypertensive rats than females (Ashton et al. 1991). These data suggest that sex steroids may regulate aldosterone secretion.
Aldosterone is synthesized from cholesterol in the zona glomerulosa of the adrenal cortex. The first step is the transfer of cholesterol to the inner mitochondrial membrane catalyzed by the Steroidogenic Acute Regulatory (StAR) protein. Then, cholesterol is converted to aldosterone by a series of enzymatic reactions catalyzed by dehydrogenases and mixed-function oxidases which take place in the mitochondria and the endoplasmic reticulum. The last step involves the conversion of 11-deoxycorticosterone to aldosterone by the enzyme aldosterone synthase in the mitochondria. Aldosterone is released into the circulation without intracellular storage so there is a close link between aldosterone biosynthesis and secretion (Stewart 2003; Payne et al. 2004; Connell et al. 2005). The calcium/calmodulin/calmodulin-dependent protein kinase (CaMK) pathway is critical in the regulation of aldosterone secretion by upregulating the expression of aldosterone synthase (Pezzi et al. 1996; Condon et al. 2002).
Taken together these data prompted us to hypothesize that at physiological concentrations, the nonaromatizable androgen dihydrotestosterone (DHT) stimulates and estradiol decreases aldosterone secretion by human adrenal cells. To test this hypothesis we used human adrenocortical cell line H295R, which is the only adrenal cell line that expresses all of the steroidogenic enzymes required for the synthesis of aldosterone from cholesterol, has a steroid secretion pattern and regulation similar to that of primary adrenal cell cultures, and has been proven to be an excellent model to study adrenal cell physiology (Rainey et al. 1994; Rainey et al. 2004). Furthermore, it has already been reported that H295R cells express functional androgen, and α- and β-estradiol receptors (Rossi et al. 1998; Montanaro et al. 2005).
Contrary to our hypothesis, only supraphysiological concentrations of DHT stimulated aldosterone secretion by H295R human adrenocortical cells. Since there is a remarkable increment in the use of high doses of androgens (Bhasin et al. 1996; Rhoden et al. 2004), such as in hormone replacement therapy in the elderly, in recreational and professional body-builders and in female-to-male conversion in transsexual individuals, we decided to perform further studies to elucidate the pathways involved in DHT mediated aldosterone secretion.
MATERIALS AND METHODS
Materials
Angiotensin II (Ang II) was obtained from American Peptide Company Inc. (Sunnyvale, CA). Bisindolylmaleimide I (GF 109203X) and Gö 6983 were from EMD Biosciences (San Diego, CA). W-7 was obtained from Tocris (Ellisville, MO). Human adrenal total RNA (pooled from 61 male/female Caucasian donors, ages 15–61) was obtained from BD Biosciences (Mountain View, CA). All other chemicals were obtained from Sigma Chemical Co. (St. Louis, MO).
Cell culture
H295R human adrenocortical cells (Bird et al. 1993) were cultured in H295R complete media containing DMEM:F12 (1:1) supplemented with 2% Ultroser G (Biosepra, Villeneuve-la-Garenne, France), ITS-Plus (Discovery Labware, Bedford, MA) and antibiotic/antimycotic mixture (Invitrogen, Carlsbad, CA), as we previously described (Romero et al. 2004).
Experimental design
H295R cells were grown to subconfluence in 24-well plates. For experiments, fresh media (1 ml) containing various agents was added for an additional period of 24 h. When inhibitors were used, they were added in fresh media 30 min prior to other reagents. At the end of the incubation period, media was removed and saved for steroids determination. Cells were lysed with M-PER lysis buffer (Pierce, Rockford, IL) and protein concentration measured with the bicinchoninic acid method (Pierce) using bovine serum albumin as standard.
Effect of DHT and estradiol on basal and Ang II-stimulated aldosterone secretion
In order to determine whether androgens or estradiol modulate basal or Ang II-stimulated aldosterone secretion, human adrenal cells at subconfluence in 24-well plates were incubated with DHT (1–1000 nM) or estradiol (1–1000 nM) alone in the presence or absence of Ang II (10 nM) for 24 h. At the end of the incubation period, media was removed and saved for steroids determination. DHT was used in this study since testosterone can suffer aromatization and be enzymatically converted to estradiol. In order to determine if the DHT effect was specific for aldosterone, cortisol secretion was determined in the same cell culture supernatant. DHT and estradiol were diluted in ethanol. The final concentration of ethanol in the incubation media was 0.01 %. Control incubations included the same concentration of vehicle.
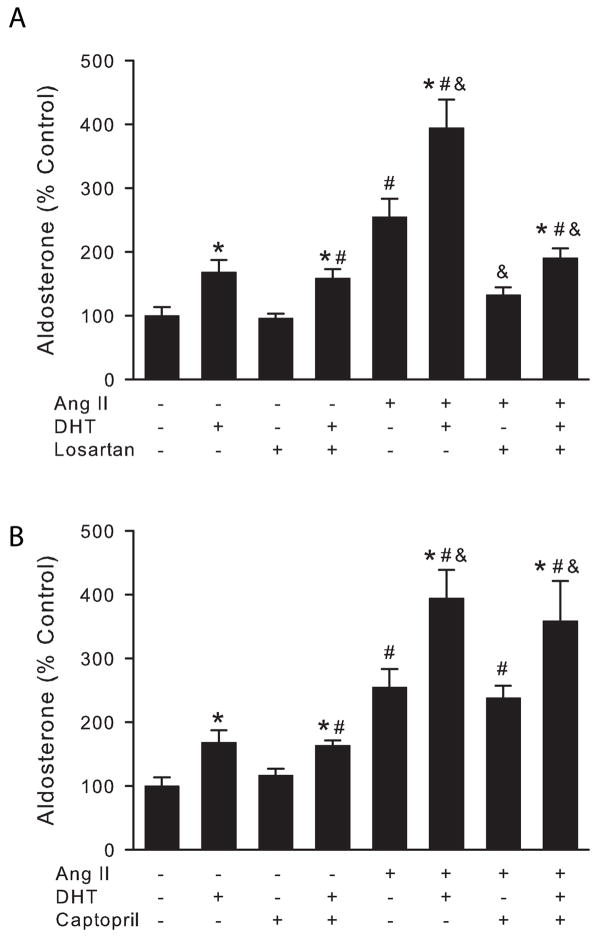
Effect of losartan or captopril (blockers of the intra-adrenal RAS) on DHT-mediated increase in aldosterone secretion
Since androgens upregulate intra-organ tissue renin-angiotensin system (RAS) and there is an intra-adrenal renin-angiotensin system which is also expressed and functional in H295R cells (Hilbers et al. 1999), we evaluated whether DHT-mediated aldosterone secretion involves the intra-adrenal renin-angiotensin system. Cell culture media was replaced with media containing the AT1 receptor antagonist losartan (10 μM), or the Angiotensin converting enzyme inhibitor (ACEI) captopril (10 μM) for 30 min prior to addition of DHT (1 μM) in the presence or absence of Ang II (10 nM). Cells were incubated and media harvested 24 later for aldosterone determination.
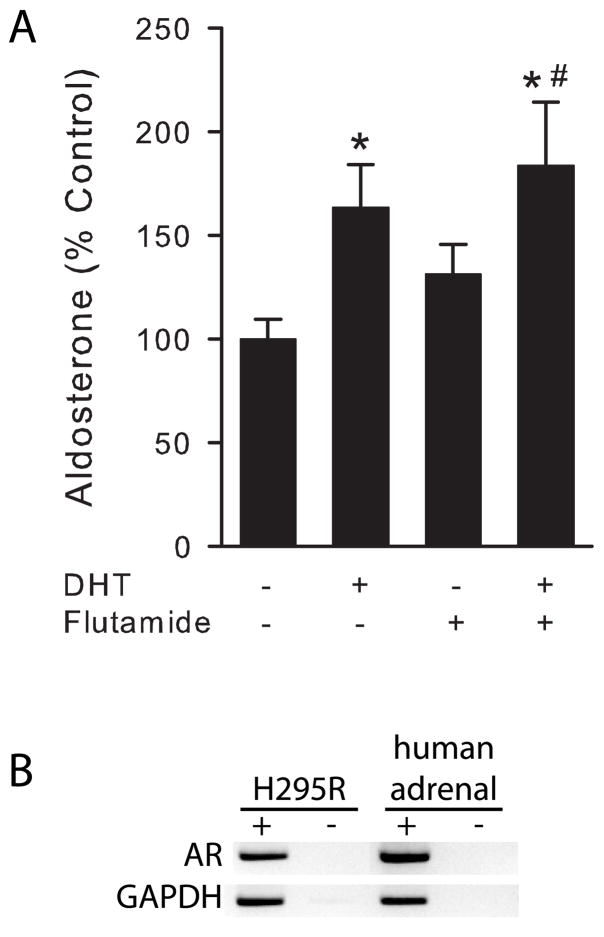
Effect of the androgen receptor antagonist flutamide on DHT-mediated aldosterone secretion
To evaluate whether the effect of DHT on aldosterone secretion from human adrenal cells was mediated via the androgen receptor, cells were incubated in the presence and absence of flutamide. Media was changed on human adrenal cells at subconfluence and flutamide (10 μM) was added to the media. Following 30 min preincubation with flutamide, DHT (1 μM) was added and media was harvested 24 h later for aldosterone determination. Flutamide concentration (10 μM) has been previously shown to effectively block androgen actions in other systems (Tep-areenan et al. 2002).
Role of calmodulin/CaMK and protein kinase C in mediating the DHT-stimulated aldosterone secretion
To evaluate the possible mechanisms by which DHT causes aldosterone secretion from human adrenal cells, cells were preincubated for 30 min with an inhibitor of the CaMK (KN-93, 5 μM), an inhibitor of calmodulin (W-7, 50 μM), an inhibitor of the protein kinase C superfamily (bisindolylmaleimide I, Bis I, 5 μM) or vehicle. Following preincubation, DHT (1 μM) was added to media and media was harvested 24 h later for aldosterone determination. Inhibitor concentrations were chosen based in previous studies, from our and other laboratories, using H295R cells, if available, or other adrenocortical cell systems (Kawamura et al. 2003; Li et al. 2003; Bassett et al. 2004; Romero et al. 2006a; Romero et al. 2007).
Steroid ELISAs
Aldosterone and cortisol secretion into the media were quantified using two in-house developed ELISAs which make use of highly specific monoclonal or polyclonal antibodies against aldosterone or cortisol, respectively, that have been characterized at length previously (Gomez-Sanchez et al. 1987;Romero et al. 2006b). Assay sensitivity was 20 pg/ml for aldosterone and 1 ng/ml for cortisol. In addition, we determined whether DHT or estradiol crossed-reacted with the antibodies to aldosterone or cortisol in the ELISA. Media containing equivalent concentrations of DHT or estradiol were incubated in the absence of cells for steroid quantification. No cross reactivity was observed with either DHT or 17β-estradiol at any concentration tested in H295R cells, confirming the specificity of the antibodies.
RNA extraction and RT-PCR for androgen receptor
Total RNA was extracted with Tri-Reagent (MRC, Cincinnati, OH), resuspended in diethyl pyrocarbonate treated H2O, DNase treated with Turbo DNA-free kit (Ambion, Austin, TX), as we previously described (Yanes et al. 2005). For reverse transcription 5 μg total RNA was incubated with 0.5 μg T12VN and Superscript III (Invitrogen, Carlsbad, CA) following the manufacturer’s suggested protocol. Human androgen receptor primers (forward: 5′-CGGAAGCTGAAGAAACTTGG-3′, reverse: 5′-ATGGCTTCCAGGACATTCAG-3′) were designed with Primer3 software (Rozen et al. 2000). GAPDH primers were previously described (Romero et al. 2004). PCR reactions were performed with 1 μl RT product, 1 μl Titanium Taq DNA polymerase (Clontech, Mountain View, CA), 0.2 mM dNTPs and 0.1 μM each primer. Cycling conditions were 1 min 95 C, 35 cycles of 15 sec 95 C, 15 sec 60 C and 1 min 72 C, followed by 10 min at 72 C. PCR products were resolved by high resolution gel electrophoresis in 4 % NuSieve 3:1 agarose gels (Cambrex, Rockland, ME).
Statistical analysis
Results are expressed as mean ± SEM. Two groups were compared by Student’s t-test, and multiple groups were analyzed by factorial ANOVA followed by Newman-Keuls’ post hoc comparisons. Statistical calculations were performed with Statistica software package version 7.1 (StatSoft, Inc., Tulsa, OK). Differences were considered significant at P < 0.05.
RESULTS
Supraphysiological concentrations of DHT increased basal and Ang II-stimulated aldosterone secretion by H295R cells
Aldosterone and cortisol are the main mineralocorticoid and glucocorticoid, respectively, secreted by adrenocortical cells. In the first series of experiments, the question as to whether sex steroid hormones could modulate the secretion of aldosterone and/or cortisol from human adrenal cells was addressed. As shown in Figure 1, H295R cells were incubated with increasing concentrations of DHT or 17β-estradiol for 24 h, in the presence or absence of Ang II, and aldosterone and cortisol secretion in cell culture supernatants was determined by ELISAs. At physiological concentrations neither DHT nor estradiol stimulated aldosterone secretion. However, DHT caused a 2-fold increase in aldosterone secretion under basal conditions and a 2.3-fold increase in aldosterone secretion under Ang II-stimulatory conditions (Fig. 1, A) at high nanomolar concentrations (300–1000 nM).
Figure 1.
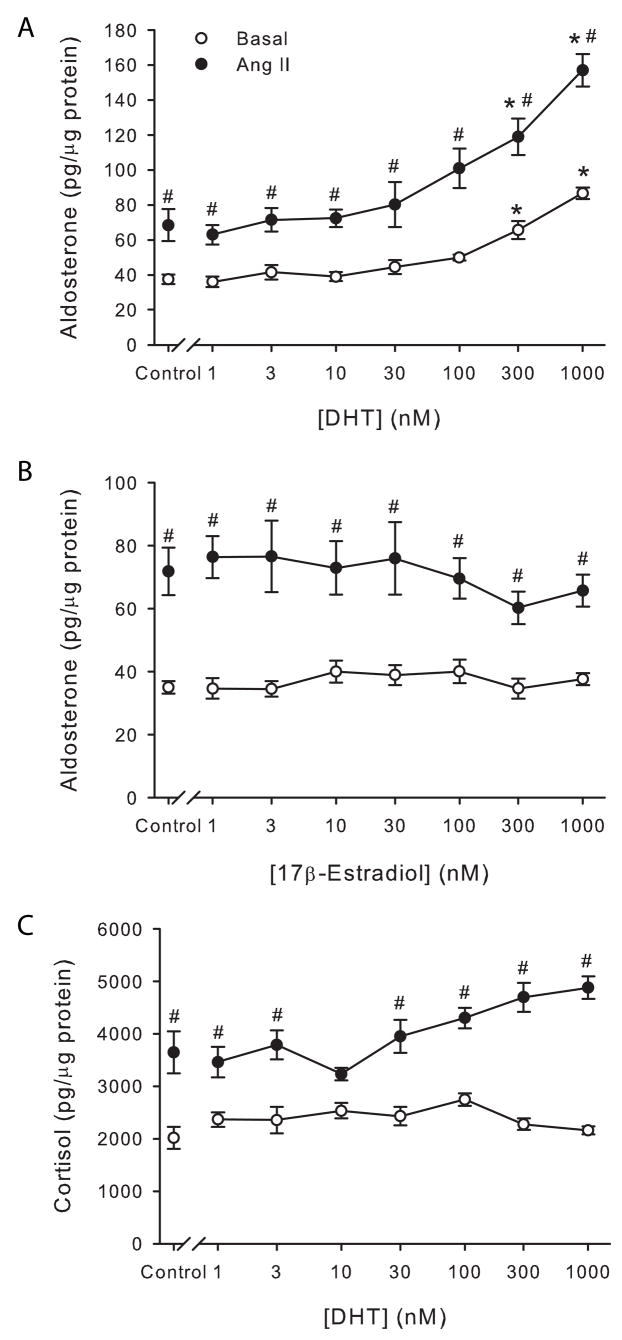
DHT, but not estradiol, stimulated basal and Angiotensin II-mediated aldosterone secretion by H295R human adrenocortical cells. H295R cells were incubated with increasing concentrations of DHT (A, C) or 17β-estradiol (B) in the presence or absence of Angiotensin II (10 nM) for 24 h. Aldosterone (A, B) or cortisol (C) concentration was measured in media supernatant and cells lysed to quantify total protein. *: P < 0.05 vs. control cells, #: P < 0.05 vs. basal condition, n = 4.
To test the specificity of the effect of DHT on aldosterone secretion, we determined the effect of DHT on cortisol secretion in the same cell culture supernatants. DHT did not significantly affect cortisol secretion at any concentration tested, nor did DHT increase cortisol in Ang II-treated cells (Fig. 1, C).
DHT-mediated aldosterone secretion is independent of the Renin Angiotensin System
Several tissues contain an endogenous RAS that functions in an autocrine or paracrine way (Lavoie et al. 2003). The adrenal gland, as well as H295R cells, expresses all the components of the RAS (Hilbers et al. 1999). Testosterone stimulates renal angiotensinogen expression in rat kidney (Ellison et al. 1989) and DHT also has been shown to stimulate renin expression in adrenal glands from mice (Wagner et al. 1990). Therefore, we tested if the DHT-mediated increase in aldosterone secretion is mediated by the local RAS. We pretreated H295R cells with the Ang II receptor type 1 (AT-1R) blocker, losartan, or the Angiotensin Converting Enzyme inhibitor, captopril, for 30 min and then incubated cells in the presence or absence of a maximal stimulatory dose of DHT (1 μM) for 24 h (Fig. 2). Losartan had no effect on DHT-mediated aldosterone secretion under basal or Ang II-stimulated conditions (Fig. 2, A). As expected, losartan abolished Ang II-mediated aldosterone secretion. Similar results were observed when H295R cells were pretreated with captopril (Fig. 2, B), although, as expected, captopril had no effect on Ang II-mediated aldosterone secretion. These results confirmed that the DHT effect on aldosterone secretion is independent of the intra-adrenal RAS.
Figure 2.
DHT-mediated aldosterone secretion is independent of the intra adrenal renin-angiotensin system. H295R cells were treated with 10 μM losartan (A), 10 μM captopril (B) or vehicle for 30 min and then incubated with 1 μM DHT or vehicle in the presence or absence of Angiotensin II (Ang II, 10 nM) for 24 h. Steroid concentration was measured in media supernatant and cells lysed to quantify total protein. *: P < 0.05 vs. no DHT under same conditions, #: P < 0.05 vs. control cells, &: P < 0.005 vs. Ang II treated cells, n = 4.
DHT-mediated aldosterone secretion is not blocked by flutamide
To test if the DHT effect on aldosterone secretion is mediated by the classical androgen receptor, we performed incubations using the androgen receptor antagonist, flutamide (Fig. 3, A). Flutamide pretreatment did not modify DHT-mediated aldosterone secretion although H295R cells used in our study did indeed express the classical androgen receptor mRNA as well as human adrenal gland (Fig. 3, B).
Figure 3.
DHT-mediated aldosterone secretion is independent of the classical androgen receptor. A, H295R cells were treated with 10 μM flutamide or vehicle for 30 min and then incubated in the presence or absence of DHT (1 μM) for 24 h. Steroid concentration was measured in media supernatant and cells lysed to quantify total protein. *: P < 0.05 vs. control cells, #: P < 0.05 vs. flutamide-treated cells, n = 6. B, Androgen receptor mRNA expression in H295R cells and human adrenal glands detected by agarose gel electrophoresis of RT-PCR reactions in the presence (+) or absence (−) of reverse transcriptase. GAPDH mRNA expression was used as control. Representative gel from three replicates.
Calmodulin/CaMK and PKC are involved in DHT-mediated aldosterone secretion
To study intracellular signaling mechanisms by which DHT stimulates aldosterone secretion from human adrenal cells, we performed the following experiments. To study if the CaMK is involved in DHT-mediated aldosterone secretion, H295R cells were pre-incubated for 30 min with the CaMK inhibitor KN-93. KN-93 significantly reduced DHT-mediated aldosterone secretion (Fig. 4, A). To further confirm that the CaMK is involved in DHT-mediated aldosterone secretion, we studied the role of calmodulin which is a required activator of CaMK. We pretreated H295R cells with the calmodulin antagonist W-7 and then incubated them in the presence or absence of DHT. W-7 completely abolished DHT-mediated increases in aldosterone secretion (Fig. 4, B), confirming our previous results indicating that the calmodulin/CaMK signaling pathway is indeed involved in DHT-mediated aldosterone secretion.
Figure 4.
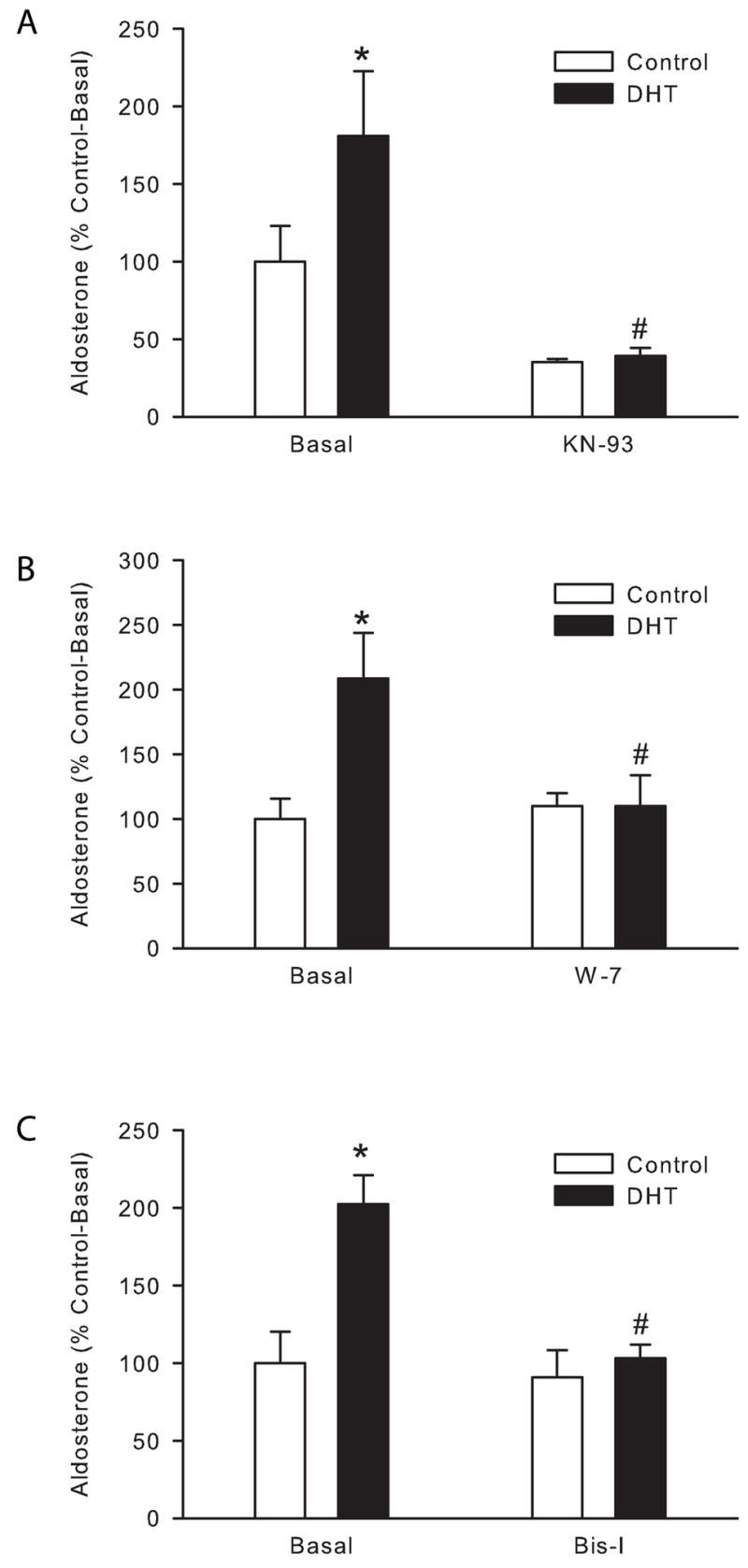
DHT-mediated aldosterone secretion acts through the calmodulin/calmodulin dependent kinase and PKC pathways. H295R cells were treated with 50 μM W-7 (A), 5 μM KN-93 (B), 5 μM Bisindolylmaleimide I (Bis I, C) or vehicle for 30 min and then incubated in the presence or absence of DHT (1 μM) for 24 h. Steroid concentration was measured in media supernatant and cells lysed to quantify total protein. *: P < 0.05 vs. control cells, #: P < 0.05 vs. DHT-treated cells, n = 4.
PKC is a superfamily of more than 9 protein kinases. To determine whether PKC is involved in DHT-mediated aldosterone secretion, H295R cells were incubated with the PKC superfamily inhibitor, Bisindolylmaleimide I (Bis-I). Bis-I completely abolished DHT-mediated increase in aldosterone secretion by H295R cells (Fig. 4, C). These results indicate that PKC also mediates DHT-mediated increase in aldosterone secretion by adrenal cells.
DISCUSSION
The main findings in this report are that: 1) supraphysiological concentrations of the nonaromatizable androgen DHT increased basal and Ang II-stimulated aldosterone secretion by H295R human adrenocortical cells; 2) physiological concentrations sex steroids (DHT and estradiol) did not modify aldosterone secretion by H295R cells; 3) the stimulatory effect of DHT is not blocked by flutamide; and 4) the calmodulin/CaMK and PKC intracellular signaling pathways are involved in DHT-mediated aldosterone secretion.
Several human and animal studies suggest that sex steroids may alter aldosterone secretion levels. For example, under strict control of sodium intake, men have higher levels of plasma aldosterone than women (Miller et al. 1999). It has also been shown that estradiol decreases ACTH- and Ang II-mediated aldosterone secretion in rats (Roesch et al. 2000). Those data prompted us to hypothesize that sex steroids will regulate aldosterone secretion by the human adrenal gland. Contrary to our original hypothesis, physiological concentrations of DHT or estradiol did not modify aldosterone secretion by H295R cells. However, DHT at supraphysiological concentrations stimulated basal and Ang II-mediated aldosterone secretion. Since there is a remarkable increment in the use of supraphysiological doses of androgens (Bhasin et al. 1996; Rhoden et al. 2004), such as in hormone replacement therapy in the elderly, in recreational and professional body-builders and in female-to-male conversion in transsexual individuals our observations could have significant clinical relevance.
Supraphysiologcial levels of plasma testosterone correlate with increased cardiovascular diseases. Concentrations of 300 nM testosterone are often observed in professional and recreational body-builders under androgen supplements, and this population is prone to suffer from cardiovascular diseases (Bardin 1996; Bhasin et al. 1996). High levels of androgens are observed also in women with polycystic ovary syndrome, a condition which commonly presents with cardiovascular diseases (Chen et al. 2007). These populations of patients present elevated plasma aldosterone levels (Cascella et al. 2006). Furthermore, during the last decade an increasing number of both elderly men and women are being administered androgen supplements to increase their libido and well being. In all these conditions the presence of high levels of testosterone may mediate increases in plasma aldosterone that ultimately will promote hypertension, cardiovascular diseases and target organ damage.
It is very well known that androgens exert both genomic and non-genomic actions (Roy et al. 1999; Heinlein et al. 2002; Boonyaratanakornkit et al. 2007). Genomic actions of androgens are mediated through the classic androgen receptor, which is a 110 kDa protein composed of multiple domains for ligand (i.e. androgen) binding, DNA binding and transactivation. Ligand-bound classic androgen receptor mainly functions as a transcription factor modulating the expression of androgen receptor target genes. On the other hand, non-genomic actions of androgens include increase in intracellular calcium, and protein kinases activation such as Src tyrosine kinase (c-Src), extracellular signal-regulated kinase 1/2 (ERK 1/2) and phosphatidylinositol 3-kinase (PI3K) (Migliaccio et al. 2000; Kousteni et al. 2001; Guo et al. 2002; Sun et al. 2003; Nguyen et al. 2005; Sun et al. 2006). In our present study we have found that flutamide, a classical androgen receptor blocker, did not modify DHT-mediated aldosterone secretion. Although these data may suggest that the action of DHT upon aldosterone secretion is non-genomic, the differentiation between non-genomic vs. genomic effects is much more complex and cannot been firmly concluded from current experimental data. For example, androgen non-genomic actions are rapid, but they can have long-lasting effects since all the second messenger pathways mentioned above can ultimately mediate transcription regulatory effects. More studies need to be done in other to clarify the nature of the DHT stimulatory effect upon aldosterone secretion.
Several tissues contain an endogenous RAS that functions in an autocrine or paracrine way (Lavoie et al. 2003). The adrenal gland, as well as H295R cells, expresses all the components of the RAS (Hilbers et al. 1999). Testosterone stimulates renal angiotensinogen expression in rat kidney (Ellison et al. 1989) and DHT also has been shown to stimulate renin expression in adrenal glands from mice (Wagner et al. 1990). However, our study shows that androgens stimulate aldosterone secretion by human adrenal gland cells independently of the intra adrenal renin angiotensin system.
We then studied the intracellular signaling mechanisms by which DHT stimulates aldosterone secretion by human adrenal cells. Our studies showed that DHT-mediated aldosterone secretion is mediated by the calcium/calmodulin/CaMK pathway since it was completely blocked by the CaMK inhibitor KN-93 and the calmodulin inhibitor W-7. The intracellular signaling mechanisms by which DHT through CaMK stimulates aldosterone secretion remain unknown although we may speculate about some possible mechanisms. Aldosterone synthase promoter region contain cAMP response elements (CRE) and activating transcription factors (ATF) regulatory elements which have been shown to be important for aldosterone synthase expression regulation (Kawamoto et al. 1992; Clyne et al. 1997; Bassett et al. 2000). CaMK phosphorylates and consequently activates CRE binding (CREB) and ATF family members (Matthews et al. 1994; Sun et al. 1996; Kingsley-Kallesen et al. 1999). Furthermore, CaMK type I, and type IV to a lesser extent, have been shown to upregulate aldosterone synthase expression in H295R cells (Condon et al. 2002). These observations allow us to speculate that DHT through CaMK may activate CREB and ATF proteins which in turn could increase aldosterone synthase expression leading to increased aldosterone secretion.
PKC is a superfamily of more than 9 protein kinases. Our study shows that bisindolylmaleimide I (Bis-I), a PKC superfamily inhibitor completely abolished DHT-mediated increase in aldosterone secretion by H295R cells. It has been previously reported that Ang II activates multiple PKC isoforms in human adrenal cells (LeHoux et al. 2001; Lehoux et al. 2007). However, the role of PKC in aldosterone synthesis is controversial; PKC activation increases aldosterone secretion but also decreases aldosterone synthase expression in adrenocortical cells (Kojima et al. 1984; Hajnoczky et al. 1992; LeHoux et al. 1998; Betancourt-Calle et al. 1999; LeHoux et al. 2001; LeHoux et al. 2006). Our results indicate that PKC is involved in DHT-mediated aldosterone secretion suggesting that Ang II and DHT may be, at least partially, using common intracellular signaling pathways to regulate aldosterone secretion. However, the presence of multiple PKC isoforms, each one regulating different downstream effectors, increases the complexity of aldosterone synthesis regulation in H295R cells. For example, although PKCε decreases Ang II-mediated upregulation of aldosterone synthase expression in H295R cells (LeHoux et al. 2006), we have previously shown that Ang II activates PKCε which causes activation of protein kinase D which in turn upregulates aldosterone secretion and aldosterone synthase expression in H295R cells (Romero et al. 2006c).
In summary, our present study suggests that physiological concentrations of sex steroids failed to modified aldosterone secretion by human adrenal cells. However, supraphysiological concentrations of DHT significantly stimulated aldosterone secretion through calcium/calmodulin/CaMK and PKC intracellular signaling pathways. The stimulatory effect of DHT upon aldosterone secretion can add insight in the pathogenesis of hypertension, cardiovascular diseases and target organ damage in hyperandrogenism status, such as hormone replacement therapies with testosterone in men and women, body builders, and female-to-male transsexual individuals.
Acknowledgments
We thank Dr. W. E. Rainey (Medical College of Georgia, Augusta, GA) and Dr. C. E. Gomez-Sanchez (G.V. Montgomery VA Medical Center, Jackson, MS) for generously providing H295R cells and antibodies respectively. We thank Dr. J. F. Reckelhoff (University of Mississippi Medical Center, Jackson, MS) for critical reading of the manuscript. We thank Dr. D. B. Sittman from the Mississippi Functional Genomics Network-Genomics facility (University of Mississippi Medical Center, Jackson, MS) for the use of the facility and helpful advice.
This work was supported by a Postdoctoral Fellowship from the American Heart Association Southeast Affiliate (0425461B to LLY), by a Scientist Development Award from the American Heart Association National Center (0830239N to LLY) and National Institutes of Health Grant RR016476 from the MFGN INBRE Program of the National Center for Research Resources.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ashton N, Balment RJ. Sexual dimorphism in renal function and hormonal status of New Zealand genetically hypertensive rats. Acta Endocrinol (Copenh) 1991;124:91–97. doi: 10.1530/acta.0.1240091. [DOI] [PubMed] [Google Scholar]
- Baker L, Meldrum KK, Wang M, Sankula R, Vanam R, Raiesdana A, Tsai B, Hile K, Brown JW, Meldrum DR. The role of estrogen in cardiovascular disease. J Surg Res. 2003;115:325–344. doi: 10.1016/s0022-4804(03)00215-4. [DOI] [PubMed] [Google Scholar]
- Bardin CW. The anabolic action of testosterone. N Engl J Med. 1996;335:52–53. doi: 10.1056/NEJM199607043350111. [DOI] [PubMed] [Google Scholar]
- Bassett MH, Suzuki T, Sasano H, White PC, Rainey WE. The orphan nuclear receptors NURR1 and NGFIB regulate adrenal aldosterone production. Mol Endocrinol. 2004;18:279–290. doi: 10.1210/me.2003-0005. [DOI] [PubMed] [Google Scholar]
- Bassett MH, Zhang Y, White PC, Rainey WE. Regulation of human CYP11B2 and CYP11B1: comparing the role of the common CRE/Ad1 element. Endocrine Research. 2000;26:941–951. doi: 10.3109/07435800009048620. [DOI] [PubMed] [Google Scholar]
- Betancourt-Calle S, Bollag WB, Jung EM, Calle RA, Rasmussen H. Effects of angiotensin II and adrenocorticotropic hormone on myristoylated alanine-rich C-kinase substrate phosphorylation in glomerulosa cells. Mol Cell Endocrinol. 1999;154:1–9. doi: 10.1016/s0303-7207(99)00111-2. [DOI] [PubMed] [Google Scholar]
- Bhasin S, Storer TW, Berman N, Callegari C, Clevenger B, Phillips J, Bunnell TJ, Tricker R, Shirazi A, Casaburi R. The effects of supraphysiologic doses of testosterone on muscle size and strength in normal men. N Engl J Med. 1996;335:1–7. doi: 10.1056/NEJM199607043350101. [DOI] [PubMed] [Google Scholar]
- Bird IM, Hanley NA, Word RA, Mathis JM, McCarthy JL, Mason JI, Rainey WE. Human NCI-H295 adrenocortical carcinoma cells: a model for angiotensin-II-responsive aldosterone secretion. Endocrinology. 1993;133:1555–1561. doi: 10.1210/endo.133.4.8404594. [DOI] [PubMed] [Google Scholar]
- Boonyaratanakornkit V, Edwards DP. Receptor mechanisms mediating non-genomic actions of sex steroids. Semin Reprod Med. 2007;25:139–153. doi: 10.1055/s-2007-973427. [DOI] [PubMed] [Google Scholar]
- Cascella T, Palomba S, Tauchmanova L, Manguso F, Di Biase S, Labella D, Giallauria F, Vigorito C, Colao A, Lombardi G, Orio F. Serum aldosterone concentration and cardiovascular risk in women with polycystic ovarian syndrome. J Clin Endocrinol Metab. 2006;91:4395–4400. doi: 10.1210/jc.2006-0399. [DOI] [PubMed] [Google Scholar]
- Chen MJ, Yang WS, Yang JH, Chen CL, Ho HN, Yang YS. Relationship Between Androgen Levels and Blood Pressure in Young Women With Polycystic Ovary Syndrome. Hypertension. 2007;49:1442–1447. doi: 10.1161/HYPERTENSIONAHA.106.083972. [DOI] [PubMed] [Google Scholar]
- Clyne CD, Zhang Y, Slutsker L, Mathis JM, White PC, Rainey WE. Angiotensin II and potassium regulate human CYP11B2 transcription through common cis-elements. Mol Endocrinol. 1997;11:638–649. doi: 10.1210/mend.11.5.9920. [DOI] [PubMed] [Google Scholar]
- Condon JC, Pezzi V, Drummond BM, Yin S, Rainey WE. Calmodulin-dependent kinase I regulates adrenal cell expression of aldosterone synthase. Endocrinology. 2002;143:3651–3657. doi: 10.1210/en.2001-211359. [DOI] [PubMed] [Google Scholar]
- Connell JM, Davies E. The new biology of aldosterone. J Endocrinol. 2005;186:1–20. doi: 10.1677/joe.1.06017. [DOI] [PubMed] [Google Scholar]
- Dean SA, Tan J, O’Brien ER, Leenen FH. 17beta-estradiol downregulates tissue angiotensin-converting enzyme and ANG II type 1 receptor in female rats. Am J Physiol Regul Integr Comp Physiol. 2005;288:R759–766. doi: 10.1152/ajpregu.00595.2004. [DOI] [PubMed] [Google Scholar]
- Ellison KE, Ingelfinger JR, Pivor M, Dzau VJ. Androgen regulation of rat renal angiotensinogen messenger RNA expression. J Clin Invest. 1989;83:1941–1945. doi: 10.1172/JCI114102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Expert Panel on Detection Evaluation, Treatment of High Blood Cholesterol in Adults. Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III) JAMA. 2001;285:2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- Gomez-Sanchez CE, Foecking MF, Ferris MW, Chavarri MR, Uribe L, Gomez-Sanchez EP. The production of monoclonal antibodies against aldosterone. Steroids. 1987;49:581–587. doi: 10.1016/0039-128x(87)90097-3. [DOI] [PubMed] [Google Scholar]
- Guo Z, Benten WP, Krucken J, Wunderlich F. Nongenomic testosterone calcium signaling. Genotropic actions in androgen receptor-free macrophages. J Biol Chem. 2002;277:29600–29607. doi: 10.1074/jbc.M202997200. [DOI] [PubMed] [Google Scholar]
- Hajnoczky G, Varnai P, Buday L, Farago A, Spat A. The role of protein kinase-C in control of aldosterone production by rat adrenal glomerulosa cells:activation of protein kinase-C by stimulation with potassium. Endocrinology. 1992;130:2230–2236. doi: 10.1210/endo.130.4.1547736. [DOI] [PubMed] [Google Scholar]
- Heinlein CA, Chang C. The roles of androgen receptors and androgen-binding proteins in nongenomic androgen actions. Mol Endocrinol. 2002;16:2181–2187. doi: 10.1210/me.2002-0070. [DOI] [PubMed] [Google Scholar]
- Hilbers U, Peters J, Bornstein SR, Correa FMA, Jöhren O, Saavedra JM, Ehrhart-Bornstein M. Local renin-angiotensin system is involved in K+-induced aldosterone secretion from human adrenocortical NCI-H295 cells. Hypertension. 1999;33:1025–1030. doi: 10.1161/01.hyp.33.4.1025. [DOI] [PubMed] [Google Scholar]
- Ji H, Menini S, Mok K, Zheng W, Pesce C, Kim J, Mulroney S, Sandberg K. Gonadal steroid regulation of renal injury in renal wrap hypertension. Am J Physiol Renal Physiol. 2005;288:F513–520. doi: 10.1152/ajprenal.00032.2004. [DOI] [PubMed] [Google Scholar]
- Kawamoto T, Mitsuuchi Y, Toda K, Yokoyama Y, Miyahara K, Miura S, Ohnishi T, Ichikawa Y, Nakao K, Imura H, Ulick S, Shizuta Y. Role of steroid 11β-hydroxylase and steroid 18-hydroxylase in the biosynthesis of glucocorticoids and mineralocorticoids in humans. Proc Natl Acad Sci USA. 1992;89:1458–1462. doi: 10.1073/pnas.89.4.1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamura M, Terasaka O, Ebisawa T, Kondo I, Masaki E, Ahmed A, Kagata M. Integrity of actin-network is involved in uridine 5′-triphosphate evoked store-operated Ca2+ entry in bovine adrenocortical fasciculata cells. J Pharmacol Sci. 2003;91:23–33. doi: 10.1254/jphs.91.23. [DOI] [PubMed] [Google Scholar]
- Kingsley-Kallesen ML, Kelly D, Rizzino A. Transcriptional regulation of the transforming growth factor-beta2 promoter by cAMP-responsive element-binding protein (CREB) and activating transcription factor-1 (ATF-1) is modulated by protein kinases and the coactivators p300 and CREB-binding protein. J Biol Chem. 1999;274:34020–34028. doi: 10.1074/jbc.274.48.34020. [DOI] [PubMed] [Google Scholar]
- Kojima I, Kojima K, Kreutter D, Rasmussen H. The temporal integration of the aldosterone secretory response to angiotensin occurs via two intracellular pathways. J Biol Chem. 1984;259:14448–14457. [PubMed] [Google Scholar]
- Kousteni S, Bellido T, Plotkin LI, O’Brien CA, Bodenner DL, Han L, Han K, DiGregorio GB, Katzenellenbogen JA, Katzenellenbogen BS, Roberson PK, Weinstein RS, Jilka RL, Manolagas SC. Nongenotropic, sex-nonspecific signaling through the estrogen or androgen receptors: dissociation from transcriptional activity. Cell. 2001;104:719–730. [PubMed] [Google Scholar]
- Lavoie JL, Sigmund CD. Minireview: overview of the renin-angiotensin system--an endocrine and paracrine system. Endocrinology. 2003;144:2179–2183. doi: 10.1210/en.2003-0150. [DOI] [PubMed] [Google Scholar]
- LeHoux JG, Dupuis G, Lefebvre A. Control of CYP11B2 gene expression through differential regulation of its promoter by atypical and conventional protein kinase C isoforms. J Biol Chem. 2001;276:8021–8028. doi: 10.1074/jbc.M009495200. [DOI] [PubMed] [Google Scholar]
- LeHoux JG, Lefebvre A. Transcriptional activity of the hamster CYP11B2 promoter in NCI-H295 cells stimulated by angiotensin II, potassium, forskolin and bisindolylmaleimide. J Mol Endocrinol. 1998;20:183–191. doi: 10.1677/jme.0.0200183. [DOI] [PubMed] [Google Scholar]
- LeHoux JG, Lefebvre A. Novel protein kinase C-epsilon inhibits human CYP11B2 gene expression through ERK1/2 signalling pathway and JunB. J Mol Endocrinol. 2006;36:51–64. doi: 10.1677/jme.1.01908. [DOI] [PubMed] [Google Scholar]
- Lehoux JG, Lefebvre A. Angiotensin II activates p44/42 MAP kinase partly through PKCepsilon in H295R cells. Mol Cell Endocrinol. 2007;265–266:121–125. doi: 10.1016/j.mce.2006.12.027. [DOI] [PubMed] [Google Scholar]
- Li J, Feltzer RE, Dawson KL, Hudson EA, Clark BJ. Janus kinase 2 and calcium are required for angiotensin II-dependent activation of steroidogenic acute regulatory protein transcription in H295R human adrenocortical cells. J Biol Chem. 2003;278:52355–52362. doi: 10.1074/jbc.M305232200. [DOI] [PubMed] [Google Scholar]
- Liu PY, Death AK, Handelsman DJ. Androgens and cardiovascular disease. Endocr Rev. 2003;24:313–340. doi: 10.1210/er.2003-0005. [DOI] [PubMed] [Google Scholar]
- Matthews RP, Guthrie CR, Wailes LM, Zhao X, Means AR, McKnight GS. Calcium/calmodulin-dependent protein kinase types II and IV differentially regulate CREB-dependent gene expression. Mol Cell Biol. 1994;14:6107–6116. doi: 10.1128/mcb.14.9.6107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendelsohn ME, Karas RH. The protective effects of estrogen on the cardiovascular system. N Engl J Med. 1999;340:1801–1811. doi: 10.1056/NEJM199906103402306. [DOI] [PubMed] [Google Scholar]
- Migliaccio A, Castoria G, Di Domenico M, de Falco A, Bilancio A, Lombardi M, Barone MV, Ametrano D, Zannini MS, Abbondanza C, Auricchio F. Steroid-induced androgen receptor-oestradiol receptor beta-Src complex triggers prostate cancer cell proliferation. EMBO J. 2000;19:5406–5417. doi: 10.1093/emboj/19.20.5406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller JA, Anacta LA, Cattran DC. Impact of gender on the renal response to angiotensin II. Kidney Int. 1999;55:278–285. doi: 10.1046/j.1523-1755.1999.00260.x. [DOI] [PubMed] [Google Scholar]
- Montanaro D, Maggiolini M, Recchia AG, Sirianni R, Aquila S, Barzon L, Fallo F, Ando S, Pezzi V. Antiestrogens upregulate estrogen receptor beta expression and inhibit adrenocortical H295R cell proliferation. J Mol Endocrinol. 2005;35:245–256. doi: 10.1677/jme.1.01806. [DOI] [PubMed] [Google Scholar]
- Muller M, van der Schouw YT, Thijssen JH, Grobbee DE. Endogenous sex hormones and cardiovascular disease in men. J Clin Endocrinol Metab. 2003;88:5076–5086. doi: 10.1210/jc.2003-030611. [DOI] [PubMed] [Google Scholar]
- Neugarten J, Acharya A, Silbiger SR. Effect of gender on the progression of nondiabetic renal disease: a meta-analysis. J Am Soc Nephrol. 2000;11:319–329. doi: 10.1681/ASN.V112319. [DOI] [PubMed] [Google Scholar]
- Nguyen TV, Yao M, Pike CJ. Androgens activate mitogen-activated protein kinase signaling: role in neuroprotection. J Neurochem. 2005;94:1639–1651. doi: 10.1111/j.1471-4159.2005.03318.x. [DOI] [PubMed] [Google Scholar]
- Payne AH, Hales DB. Overview of steroidogenic enzymes in the pathway from cholesterol to active steroid hormones. Endocr Rev. 2004;25:947–970. doi: 10.1210/er.2003-0030. [DOI] [PubMed] [Google Scholar]
- Pezzi V, Clark BJ, Ando S, Stocco DM, Rainey WE. Role of calmodulin-dependent protein kinase II in the acute stimulation of aldosterone production. J Steroid Biochem Mol Biol. 1996;58:417–424. doi: 10.1016/0960-0760(96)00052-0. [DOI] [PubMed] [Google Scholar]
- Phillips GB. Is atherosclerotic cardiovascular disease an endocrinological disorder? The estrogen-androgen paradox. J Clin Endocrinol Metab. 2005;90:2708–2711. doi: 10.1210/jc.2004-2011. [DOI] [PubMed] [Google Scholar]
- Rainey WE, Bird IM, Mason JI. The NCI-H295 cell line: a pluripotent model for human adrenocortical studies. Mol Cell Endocrinol. 1994;100:45–50. doi: 10.1016/0303-7207(94)90277-1. [DOI] [PubMed] [Google Scholar]
- Rainey WE, Saner K, Schimmer BP. Adrenocortical cell lines. Mol Cell Endocrinol. 2004;228:23–38. doi: 10.1016/j.mce.2003.12.020. [DOI] [PubMed] [Google Scholar]
- Reckelhoff JF, Zhang H, Srivastava K. Gender differences in development of hypertension in spontaneously hypertensive rats: role of the renin-angiotensin system. Hypertension. 2000;35:480–483. doi: 10.1161/01.hyp.35.1.480. [DOI] [PubMed] [Google Scholar]
- Rhoden EL, Morgentaler A. Risks of testosterone-replacement therapy and recommendations for monitoring. N Engl J Med. 2004;350:482–492. doi: 10.1056/NEJMra022251. [DOI] [PubMed] [Google Scholar]
- Rocha R, Stier CT., Jr Pathophysiological effects of aldosterone in cardiovascular tissues. Trends Endocrinol Metab. 2001;12:308–314. doi: 10.1016/s1043-2760(01)00432-5. [DOI] [PubMed] [Google Scholar]
- Roesch DM, Tian Y, Zheng W, Shi M, Verbalis JG, Sandberg K. Estradiol attenuates angiotensin-induced aldosterone secretion in ovariectomized rats. Endocrinology. 2000;141:4629–4636. doi: 10.1210/endo.141.12.7822. [DOI] [PubMed] [Google Scholar]
- Romero DG, Plonczynski M, Vergara GR, Gomez-Sanchez EP, Gomez-Sanchez CE. Angiotensin II early regulated genes in H295R human adrenocortical cells. Physiol Genomics. 2004;19:106–116. doi: 10.1152/physiolgenomics.00097.2004. [DOI] [PubMed] [Google Scholar]
- Romero DG, Plonczynski MW, Gomez-Sanchez EP, Yanes LL, Gomez-Sanchez CE. RGS2 is regulated by angiotensin II and functions as a negative feedback of aldosterone production in H295R human adrenocortical cells. Endocrinology. 2006a;147:3889–3897. doi: 10.1210/en.2005-1532. [DOI] [PubMed] [Google Scholar]
- Romero DG, Vergara GR, Zhu Z, Covington GS, Plonczynski MW, Yanes LL, Gomez-Sanchez EP, Gomez-Sanchez CE. Interleukin-8 synthesis, regulation, and steroidogenic role in H295R human adrenocortical cells. Endocrinology. 2006b;147:891–898. doi: 10.1210/en.2005-0951. [DOI] [PubMed] [Google Scholar]
- Romero DG, Welsh BL, Gomez-Sanchez EP, Yanes LL, Rilli S, Gomez-Sanchez CE. Angiotensin II-mediated protein kinase D activation stimulates aldosterone and cortisol secretion in H295R human adrenocortical cells. Endocrinology. 2006c;147:6046–6055. doi: 10.1210/en.2006-0794. [DOI] [PubMed] [Google Scholar]
- Romero DG, Zhou MY, Yanes LL, Plonczynski MW, Washington TR, Gomez-Sanchez CE, Gomez-Sanchez EP. Regulators of G-protein signaling 4 in adrenal gland: localization, regulation, and role in aldosterone secretion. J Endocrinol. 2007;194:429–440. doi: 10.1677/JOE-07-0153. [DOI] [PubMed] [Google Scholar]
- Rossi R, Zatelli MC, Valentini A, Cavazzini P, Fallo F, del Senno L. Evidence for androgen receptor gene expression and growth inhibitory effect of dihydrotestosterone on human adrenocortical cells. J Endocrinol. 1998;159:373–380. doi: 10.1677/joe.0.1590373. [DOI] [PubMed] [Google Scholar]
- Roy AK, Lavrovsky Y, Song CS, Chen S, Jung MH, Velu NK, Bi BY, Chatterjee B. Regulation of androgen action. Vitam Horm. 1999;55:309–352. doi: 10.1016/s0083-6729(08)60938-3. [DOI] [PubMed] [Google Scholar]
- Rozen S, Skaletsky H. Primer3 on the WWW for general users and for biologist programmers. Methods Mol Biol. 2000;132:365–386. doi: 10.1385/1-59259-192-2:365. [DOI] [PubMed] [Google Scholar]
- Song J, Kost CK, Jr, Martin DS. Androgens augment renal vascular responses to ANG II in New Zealand genetically hypertensive rats. Am J Physiol Regul Integr Comp Physiol. 2006;290:R1608–1615. doi: 10.1152/ajpregu.00364.2005. [DOI] [PubMed] [Google Scholar]
- Stewart PM. In: The Adrenal Cortex. Williams Textbook of Endocrinology. Larsen PR, Kronenberg HM, Melmed S, Polonsky KS, editors. Philadelphia; Elsevier: 2003. pp. 491–551. [Google Scholar]
- Struthers AD. Aldosterone in heart failure: pathophysiology and treatment. Curr Heart Fail Rep. 2004;1:171–175. doi: 10.1007/s11897-004-0005-8. [DOI] [PubMed] [Google Scholar]
- Sun M, Yang L, Feldman RI, Sun XM, Bhalla KN, Jove R, Nicosia SV, Cheng JQ. Activation of phosphatidylinositol 3-kinase/Akt pathway by androgen through interaction of p85alpha, androgen receptor, and Src. J Biol Chem. 2003;278:42992–43000. doi: 10.1074/jbc.M306295200. [DOI] [PubMed] [Google Scholar]
- Sun P, Lou L, Maurer RA. Regulation of activating transcription factor-1 and the cAMP response element-binding protein by Ca2+/calmodulin-dependent protein kinases type I, II, and IV. J Biol Chem. 1996;271:3066–3073. doi: 10.1074/jbc.271.6.3066. [DOI] [PubMed] [Google Scholar]
- Sun YH, Gao X, Tang YJ, Xu CL, Wang LH. Androgens induce increases in intracellular calcium via a G protein-coupled receptor in LNCaP prostate cancer cells. J Androl. 2006;27:671–678. doi: 10.2164/jandrol.106.000554. [DOI] [PubMed] [Google Scholar]
- Tep-areenan P, Kendall DA, Randall MD. Testosterone-induced vasorelaxation in the rat mesenteric arterial bed is mediated predominantly via potassium channels. Br J Pharmacol. 2002;135:735–740. doi: 10.1038/sj.bjp.0704522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner D, Metzger R, Paul M, Ludwig G, Suzuki F, Takahashi S, Murakami K, Ganten D. Androgen dependence and tissue specificity of renin messenger RNA expression in mice. J Hypertens. 1990;8:45–52. doi: 10.1097/00004872-199001000-00008. [DOI] [PubMed] [Google Scholar]
- Wu FC, von Eckardstein A. Androgens and coronary artery disease. Endocr Rev. 2003;24:183–217. doi: 10.1210/er.2001-0025. [DOI] [PubMed] [Google Scholar]
- Yanes LL, Romero DG, Cucchiarelli VE, Fortepiani LA, Gomez-Sanchez CE, Santacruz F, Reckelhoff JF. Role of endothelin in mediating postmenopausal hypertension in a rat model. Am J Physiol Regul Integr Comp Physiol. 2005;288:R229–233. doi: 10.1152/ajpregu.00697.2003. [DOI] [PubMed] [Google Scholar]
- Yanes LL, Sartori-Valinotti JC, Reckelhoff JF. Sex steroids and renal disease: lessons from animal studies. Hypertension. 2008;51:976–981. doi: 10.1161/HYPERTENSIONAHA.107.105767. [DOI] [PubMed] [Google Scholar]