Abstract
The stimulus-evoked response of a cortical neuron depends on both details of the afferent signal and the momentary state of the larger network in which it is embedded. Consequently, identical sensory stimuli evoke highly variable responses. Using simultaneous recordings of thalamic barreloid and/or cortical barrel neurons in the rat whisker-to-barrel pathway, we determined the extent to which the responses of pairs of cells covary on a trial-by-trial basis. In the thalamus and cortical layer IV, a substantial component of trial-to-trial variability is independent of the specific parameters of the stimulus, probed here using deflection angle. These stimulus-nonspecific effects resulted in greater-than-chance similarities in trial-averaged angular tuning among simultaneously recorded pairs of barrel neurons. Such effects were not observed among simultaneously recorded thalamic and cortical barrel neurons, suggesting strong intracortical mechanisms of synchronization. Sensory adaptation produced by prior whisker deflections reduced response magnitudes and enhanced the joint angular tuning of simultaneously recorded neurons. Adaptation also decorrelated stimulus-evoked responses, rendering trial-by-trial responses of neuron pairs less similar to each other. Adaptation-induced decorrelation coupled with sharpened joint tuning could enhance the saliency of cells within thalamus or cortex that continue to fire synchronously during ongoing tactile stimulation associated with active touch.
INTRODUCTION
Many neural circuits have a narrow time window in which synchronous or near-synchronous firing of converging excitatory inputs can summate effectively to surpass spike threshold (Mittmann et al. 2005; Pouille and Scanziani 2001; Usrey et al. 2000). Temporally coherent firing can be evoked in afferent pathways by sensory stimuli. In the visual system, optimally oriented stimuli produce more synchronous firing among similarly tuned cortical neurons than do stimuli of suboptimal orientations (Kohn and Smith 2005; Samonds et al. 2003). Stimulus-evoked firing occurs on a background of internally generated spontaneous brain activity, reflecting a complex interplay of intrinsic neuronal properties and synaptic connections in recurrent networks. Spontaneous fluctuations in neuronal excitability, shared among neurons within a network, can influence the firing of neurons to any given stimulus (Destexhe and Contreras 2006). Networkwide fluctuations in cortical activity can modulate responses to visual stimuli, as first demonstrated in anesthetized primary visual cortex (Arieli et al. 1996). A similar modulating role for background activity has been demonstrated in a variety of cortical areas (see Destexhe and Contreras 2006), including the somatosensory “barrel” cortex of both anesthetized (Hasentstaub et al. 2007; Haslinger et al. 2006) and awake rodents (Petersen et al. 2003). Shared background fluctuations may be the basis for the intriguing finding that in awake primates, somatosensory cortex neurons display significant trial-by-trial correlations in stimulus-evoked spike counts even if the cells have dissimilar tuning properties (Romo et al. 2003).
In thalamocortical circuits of the rat somatosensory system, thalamic population firing synchrony strongly engages neuronal activity in cortical layer IV (Pinto et al. 2000). Synchronous thalamic firing is produced, for example, by high-velocity deflections of the topographically appropriate whisker. Such population firing activates layer IV neurons presumably by means of spatial and temporal summation of weak but highly convergent thalamocortical inputs (Bruno and Sakmann 2006). However, even highly effective stimuli evoke responses in individual thalamic and cortical neurons that vary from trial to trial, a common finding in sensory areas of the forebrain (DeWeese et al. 2005; Kumbhani et al. 2007). Here we examine the extent to which stimulus-evoked responses occur simultaneously among individual cells and how such covariation is affected by sensory adaptation that, though reduced, is present even during normal sensory behavior (Castro-Alamancos 2004).
In this study we obtained simultaneous recordings from thalamic barreloid or cortical barrel neurons in response to whisker deflections of different angular directions. We find, as expected, that neurons having similar angular tuning are more likely to fire synchronously to a given angle of whisker deflection. However, in the cortex, close to 50% of whisker-evoked covarying neural activity is independent of stimulus angle. Such stimulus-nonspecific response modulation likely reflects a strong influence of background state. Interestingly, response covariation is reduced by whisker-deflection–induced sensory adaptation, also known to reduce evoked firing rates and enhance angular tuning in this system (Khatri and Simons 2007). Adaptation is also accompanied by an increase in joint angular tuning. In conjunction with greater response tuning, adaptation-induced decorrelation, as proposed by Barlow and Földiák (1989), could enhance the impact of cells that continue to fire synchronously during ongoing stimulation associated with active touch.
METHODS
Animals and surgical preparation
Surgical preparation and maintenance of the rats during electrophysiological recording were identical to those described previously (Khatri et al. 2004). Ten Sprague–Dawley adult female rats (200–300 g) were obtained from a commercial supplier (Harlan, Indianapolis, IN). All surgical preparation was performed under halothane anesthesia. A silastic catheter was inserted into the right jugular vein and led out from the nape of the neck for later drug delivery. A short length (∼40 mm) of polyethylene tubing was inserted into the trachea for later artificial respiration and the left femoral artery was cannulated using an angiocath catheter to measure blood pressure. After exposing the skull, small stainless steel screws were placed over left occipital and frontal cortex for electroencephalographic (EEG) recordings and a ground screw was placed over the right frontal cortex. Dental acrylic was used to attach a steel post to the skull. The post, which was used to hold the animal's head without pressure points during the rest of the experiment, permitted unimpeded access to the facial vibrissae. In cortical experiments, the bone overlying the right barrel cortex was thinned and a small (∼1-mm2) craniectomy was made. For thalamic experiments, a craniectomy was made at stereotaxic coordinates overlying the ventral posterior medial nucleus (VPM: 2.0–4.5 mm posterior, 1.5–4.0 mm lateral to bregma). The dura was incised to prevent the brain from dimpling and thus suffering compression damage due to electrode insertion. Last, an acrylic dam was constructed around the skull opening and filled with saline.
Body temperature was maintained at 37°C by a servo-controlled heating blanket (Harvard Apparatus, Holliston, MA). For neural recordings, halothane was discontinued and the rat was maintained in a lightly narcotized, sedated state by intravenous (iv) infusion of fentanyl (Sublimaze, ∼10 μg·kg−1·h−1; Janssen Biochimica, Berse, Belgium). Under these recording conditions, neurons in the thalamic reticular nucleus alternate spontaneously between burst- and relay-mode firing (Hartings et al. 2003). To prevent spontaneous contraction of facial muscles, which would prevent use of our electromechanical stimulators (see following text), neuromuscular blockade was induced with pancuronium bromide (1.6 mg·kg−1·h−1) and the animal was respired (90–100 breaths/min) using a positive-pressure ventilator. A computer continuously monitored the rat's EEG, mean arterial pressure, arterial pulse rate, and tracheal airway pressure waveform. Experiments were terminated if any of the above-cited indicators could not be maintained within normal physiological ranges (see Fraser et al. 2006), although this rarely occurred.
Recordings
Extracellular unit recordings were obtained from neurons in whisker-related cortical barrels of primary somatosensory cortex and from thalamic barreloids in VPM. All pairs of neurons had the same maximally effective whisker, i.e., the principal whisker (PW). Microelectrodes of 2- to 6-MΩ impedance were made from pulled and beveled quartz-insulated platinum–tungsten (90–10%) core fibers (60-μm shank diameter; Uwe Thomas Recording, Giessen, Germany). Electrodes were advanced using a multielectrode array microdrive (Uwe Thomas Recording) that permitted independent control of two to four recording electrodes. To insert several electrodes into the same barreloid or barrel, the brain was first mapped with a carbon-fiber microelectrode (∼1 ΜΩ) while manually brushing the whiskers. After localizing VPM or barrel cortex, two to three platinum–tungsten micoelectrodes were inserted about 100 μm apart and into the same barreloid or barrel, determined during the experiment by the presence of maximal responses to the same facial whisker. In VPM barreloids, the majority of recordings (∼75%) were obtained from well-isolated single thalamocortical units (TCUs); the remainder included two or three unit waveforms. Barrel recordings were also obtained from both single-unit and multiunit data. All single units were from regular-spike units (RSUs, presumed excitatory cells), as indicated by off-line inspection of saved waveforms. Cortical multiunit activity also consisted of RSUs, as indicated by extracellular waveform characteristics, low levels of spontaneous activity, and relatively sharp angular tuning (see Bruno and Simons 2002). We combined single-unit and multiunit data sets to obtain appropriately large sample sizes for all of the reported values in the results. In all cases recording pairs were acquired from two different electrodes. A multiunit recording site was treated as one “unit” of a pair. We also analyzed data from trigeminal ganglion cells obtained in a previous study under identical recording and anesthetic conditions (Fraser et al. 2006).
Histology
At the termination of each experiment, the rat was deeply anesthetized with sodium pentobarbital (100 mg/kg, iv) and perfused transcardially for histological processing. The cortex was cut tangentially and the thalamus was sectioned coronally. Tissue sections were reacted for cytochrome-oxidase (Land and Simons 1985) and counterstained with thionine. Cortical penetrations were followed from the pial surface, using drawings of surface vasculature made at the time of the recording. Using signs of tissue disruption, recording sites were confirmed to be localized within individual barrels. Although individual electrode tracks could not always be clearly distinguished, all data reported here are taken from paired recordings acquired during electrode penetrations within the same barrel. The recording array did not allow production of lesions as marker sites; therefore recording depth in layer IV was based on microdrive readings, which have consistently paralleled anatomically determined laminar location in our recordings (Kyriazi et al. 1996). Because of the complex geometry of thalamic barreloids, no attempt was made to identify thalamic recording sites with respect to individual barreloids. However, electrode penetrations were readily identifiable and all recording sites were confirmed as being located within VPM.
Whisker stimulation protocols
The whisker evoking the most robust response was denoted as the principal whisker (PW). As previously described (Simons and Carvell 1989), a ramp-and-hold stimulus (average velocity ≃ 125 mm/s; peak amplitude = 1 mm at 10 mm from the face; duration = 200 ms) was applied to the PW randomly in each of eight angles spanning 0 to 360ο in increments of 45ο. This stimulus, when delivered at appropriate deflection angles (directions), is effective in evoking activity in neurons at all stations within the whisker-to-barrel pathway.
To study effects of adaptation, we used a stimulation protocol identical to that used recently to study the effects of adaptation on angular tuning (Khatri and Simons 2007). Each trial consisted of three sequential ramp-and-hold deflections (as before; also see Fig. 1). The first and third ramp-and-hold deflections were presented at the same angle, which varied over 360° in 45° increments as before. Inserted between them was an adapting stimulus that was presented at a caudal angle (0ο). Specifically, the first ramp-and-hold deflection was followed 500 ms later by an adapting sequence; the 500-ms period is sufficient to allow recovery of effects produced by the first ramp-and-hold deflection (Khatri and Simons 2007). The adapting sequence consisted of an onset ramp, identical to the first and third, which was followed immediately by a positively rectified 20-Hz sinusoid of 125-ms duration (2.5 cycles). The frequency of 20 Hz was chosen because this stimulus reliably adapts barrel neurons, but does not eliminate their stimulus-evoked responses (Khatri and Simons 2007; Khatri et al. 2004). The third ramp-and-hold deflection followed the end of the adapting sinusoid by 25 ms.
FIG. 1.
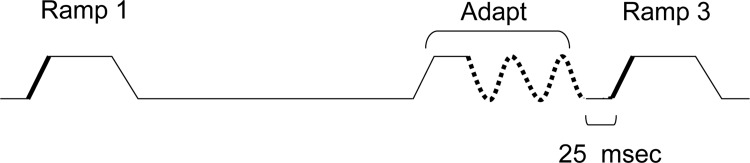
The adaptation paradigm. A nonadapted ramp is presented in one of 8 directions (0–315°, 45° increments). After a period of 500 ms, an adapting stimulus is presented in the 0° direction. Another ramp-and-hold is delivered 25 ms later. on responses (indicated by thick black lines) evoked by the onset ramps, pre- and postadaptation were compared. The stimulus has been drawn to scale, both in amplitude and time.
Neuronal adaptation effects were determined by comparing responses evoked by the first deflection in the trial to responses evoked by the third ramp-and-hold deflection; for example, smaller responses relative to those evoked by the first deflection indicate suppression induced by the adapting (second) ramp and 20-Hz stimulus. For the adapting sinusoid, only 0ο (caudal) deflections were used, inasmuch as we previously found (Khatri and Simons 2007) that adapting whisking deflections evoke angularly nonspecific suppression in both thalamic barreloid and cortical barrel neurons. Each of the eight stimulus conditions was presented with intertrial intervals of 2 s and delivered in pseudorandom order during 20 blocks. Whisker stimuli were controlled by a PC running LabVIEW (National Instruments, Austin, TX); the computer also acquired spike waveforms that were saved for later off-line inspection.
Data analysis
Unit responses were quantified by initially binning spikes with a 1-ms resolution. The response magnitude evoked by the rising phase of a ramp (on response) was calculated by taking the average number of spikes per stimulus occurring during a 20-ms period beginning 4 ms after the onset of the ramp in the thalamus and 5 ms in the cortex. These epochs capture the transient neural response to stimulus onset. For thalamic neurons, we additionally analyzed spike counts during the first 5 ms after the onset of the thalamic population response (see Khatri and Simons 2007) because firing during this time epoch is known to robustly predict the responses of cortical barrel neurons (Pinto et al. 2000).
Angular tuning
The multiangle stimulus allowed us to examine each neuron's responses on a trial-by-trial basis and on the basis of the average across multiple repetitions of the same (randomly delivered) deflection angle. A unit's angular tuning was quantified by calculating mean on response spike counts for each of the eight deflection angles and using these trial-averaged values to construct polar plots. Quantitative pairwise comparisons of polar plot shapes were made by calculating Pearson's correlation coefficients of corresponding mean on responses of the two units at each of the eight deflections angles (see Bruno et al. 2003). This yields a value, called a similarity index (SI), having a range of −1.0 to 1.0: a value of 1.0 indicates that both polar plots are identically shaped (similarly tuned), whereas −1.0 indicates the polar plots are oppositely shaped.
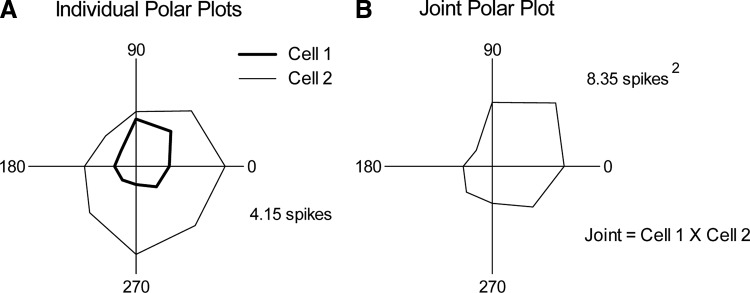
In addition to computing the angular tuning of individual units, we calculated joint angular tuning, a measure of the joint spiking behavior of neurons for each of the eight different directions. The trial-by-trial joint angular tuning of neuron pairs was quantified by taking the product of on response spike counts for each stimulus presentation and then summing across all repetitions of the same angle (see Fig. 2 for an example). A joint angular tuning index was computed by finding the value of the maximal angle response and dividing this by the mean across all eight angles. Similar max/mean indices for individual polar plots have been used to quantify angular tuning in this system (e.g., Bruno and Simons 2002). We additionally quantified the tuning of individual units and of joint firing using vector strength, a measure derived from the geometric shape of the polar plot (Khatri and Simons 2007), and obtained similar results. Here, we present only max/mean indices.
FIG. 2.
Calculation of joint angular tuning. A: trial-averaged polar plots for 2 individual members of a cell pair. B: joint tuning polar plots constructed on a trial-by-trial basis, by multiplying the spike count of cell 1 with that of cell 2 and then summing across repetitions of the same angle. Spike counts of each plot indicate the magnitude of the maximal response.
Response covariation (RC)
Simultaneous variations in the responses of pairs of neurons were quantified by determining to what degree the number of spikes evoked in both units covaried from one stimulus presentation to the next during which deflection angle, which was varied randomly within each block of trials, could change or remain the same. The RC measure differs from trial-by-trial joint angular tuning, inasmuch as RC quantifies trial-to-trial fluctuations regardless of deflection angle, whereas joint-angular tuning explicitly accounts for it. A cell pair could have a relatively large value for trial-by-trial joint-angular tuning if both cells fired at the same preferred angle(s), but a relatively small RC value if responses at other angles varied between the cells in a nonsystematic fashion. To compute RC, on response spike counts for each stimulus presentation were correlated using a Pearson's correlation coefficient, as done in previous studies that similarly quantified covarying neural activity in simultaneously recorded neurons (Kohn and Smith 2005; Romo et al. 2003)
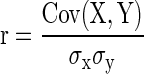 |
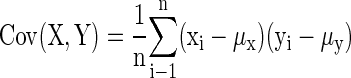 |
where x is the spike count for cell 1, y is the spike count for cell 2, i is the trial number, n is the total number of trials, and μ is the average spike count across all trials.
As noted earlier, we measured spike counts over brief time epochs because they capture the transient on response to stimulus onsets characteristic of vibrissa-activated neurons. Studies that use cross-correlation analyses of spike trains to quantify stimulus-evoked firing synchrony also typically derive measures of correlations over time windows that are longer than the 1-ms bins used to measure spike times. Such analyses, however, require large numbers of spikes, often generated by continuous, extended periods of stimulation. Our stimuli are brief and evoke relatively few spikes per stimulus. The RC measure and the stimuli used here are advantageous, however, because they are sensitive to changes in background activity, which may occur over short timescales.
A desirable feature of the RC measure is that it explicitly includes data from null trials, i.e., those in which 0 spikes are evoked. With linear regressions, we found that RC is insensitive to the spontaneous activities of unit pairs in our sample and to overall evoked firing rates (on response magnitudes). For these analyses we computed measures of joint firing by calculating the product of the mean spike counts during prestimulus (spontaneous activity) or deflection onset (on) time epochs and regressing these against each cell pair's RC value. We further assessed the robustness of the RC measure by correlating the spike count for one cell in a pair on trial i with the other cell's spike count on trial i + 1. Because stimulus angle is randomized during the experiment, this shuffling procedure preserves the firing rates and variances for the individual members of a cell pair, but eliminates the angle-specific effects of the stimuli. For all cell pairs, RC values from shuffled trials did not differ from 0.0, indicating that the RC measure is robust to differences in the overall firing rates and response variability of individual cells.
Statistical treatment of data
Prior to executing statistical tests, the normality of data was assessed. Because data points were not normally distributed, statistical comparisons of group means were made using either Mann–Whitney test or Wilcoxon's signed-rank tests. Descriptive measures are in the form of mean ± SE. Correlations were assessed with the Pearson's correlation coefficient. In certain cases, box-and-whisker plots were used to represent the data. The horizontal line within a box represents the median. The upper and lower edges of the box define the 25th and 75th percentiles and the brackets extending below and above the box (i.e., the whiskers) indicate the 10th and 90th percentiles.
RESULTS
Here we examine the extent to which trial-by-trial covariation in evoked spike counts of simultaneously recorded neurons is determined by stimulus-specific versus stimulus-nonspecific effects. Stimuli were randomized with respect to deflection angle, changing from one stimulus presentation to the next. Thus one source of trial-by-trial response covariation is the angle of the whisker deflection relative to each cell's angular tuning; this is stimulus-specific covariation. Additionally, trial-by-trial response covariation can reflect momentary changes in neuronal excitability at the time of the stimulus; this is a stimulus-nonspecific effect. Stimulus-specific and stimulus-nonspecific components of response covariation (RC) were assessed for 19 simultaneously recorded pairs in 38 (paired) recording sites within the same thalamic barreloid and for 41 pairs from 57 sites within the same cortical barrel. For the thalamic recordings, a unit was used in only one pairing, whereas for the cortical data, 25 were used once and the remainder twice (n = 16).
Angular tuning similarity and response covariation
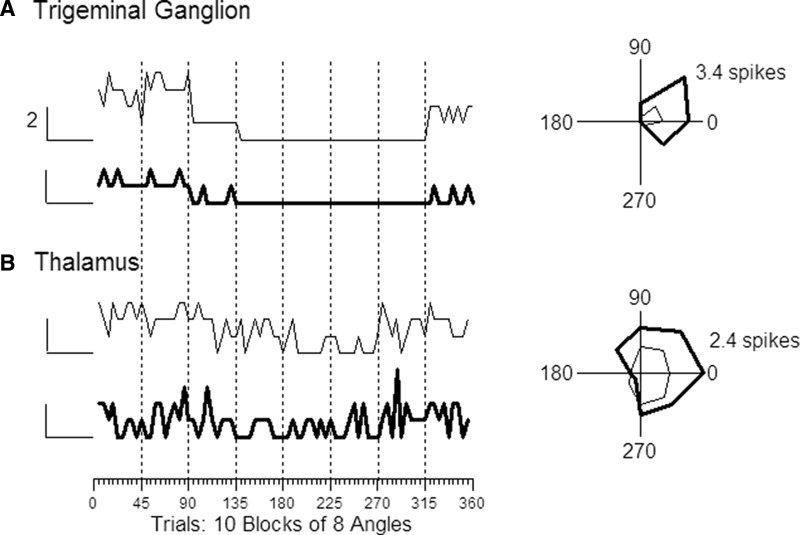
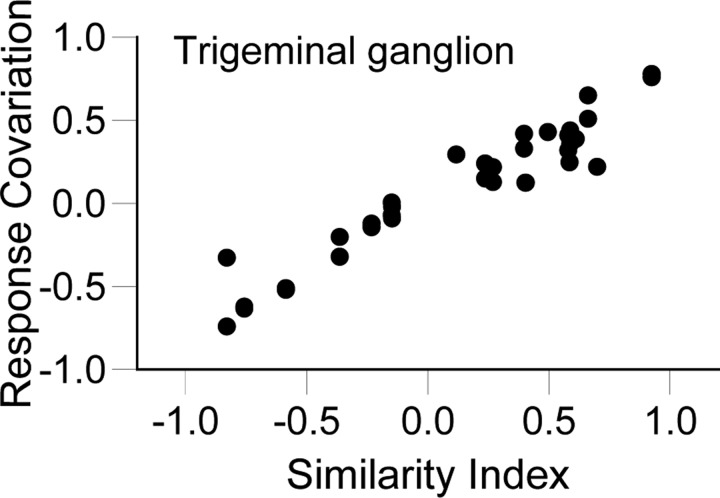
If RC is solely determined by stimulus direction, cells having similar angular tuning would be expected to fire together to a greater extent than dissimilarly tuned cells. For example, whisker-evoked responses of primary afferent neurons are highly reliable from one trial to the next (Jones et al. 2004) and the cells are strongly tuned for stimulus angle (Lichtenstein et al. 1990); on response magnitudes thus depend almost entirely on deflection angle. We examined trigeminal cells recorded one at a time to establish a baseline of expected RC values in the case of no stimulus-dependent covariation. Figure 3A shows trial-by-trial spike counts and polar plots for two trigeminal ganglion cells selected for illustration because they have similar angular tuning, as quantified by the similarity index (SI = 0.92). Although the units were recorded at different times and had different overall firing rates, their responses covary strongly (RC = 0.78) when trials are ordered by deflection angle. RC should be small for pairs of neurons having different angular preferences and large for similarly tuned pairs. This relationship is shown in Fig. 4 where RC values are plotted as a function of SI. Within the sample of 10 neurons, cells were randomly selected to be members of 45 unique pairs. For each pair, we computed the RC and compared this with the SI value. The correlation between response covariation and SI is nearly perfect and the slope of the function is steep (r = 0.96, slope = 0.72 ± 0.03). The slope of the regression line was 0.0 when the same pairs were used but the spike counts of one cell were shifted one trial forward (shuffled).
FIG. 3.
Trial-by-trial responses (left) and trial-averaged polar plots (right) for a pair of trigeminal ganglion cells (A) and a pair of thalamocortical single units (B). Although stimuli were delivered pseudorandomly with respect to deflection angle (see methods), same-angle deflections are plotted successively (n = 10) at each of the 8 deflection angles; data points are connected by solid lines for ease of visualization. Vertical scale = 2 spikes applies to all 4 traces; baseline (0 spikes) is indicated by the short horizontal bar to the left of each trace. Orientation of polar plots: 0° = caudal, 90° = dorsal. The more responsive trigeminal ganglion cell fired an average of 3.4 spikes with 45° deflections; the more responsive thalamic cell fired 2.4 spikes at 0°.
FIG. 4.
Trigeminal ganglion: similarity index (SI) vs. response covariation (RC). The relationship between SI and RC in trigeminal ganglion cell pairs is nearly perfect (r = 0.96, slope = 0.72).
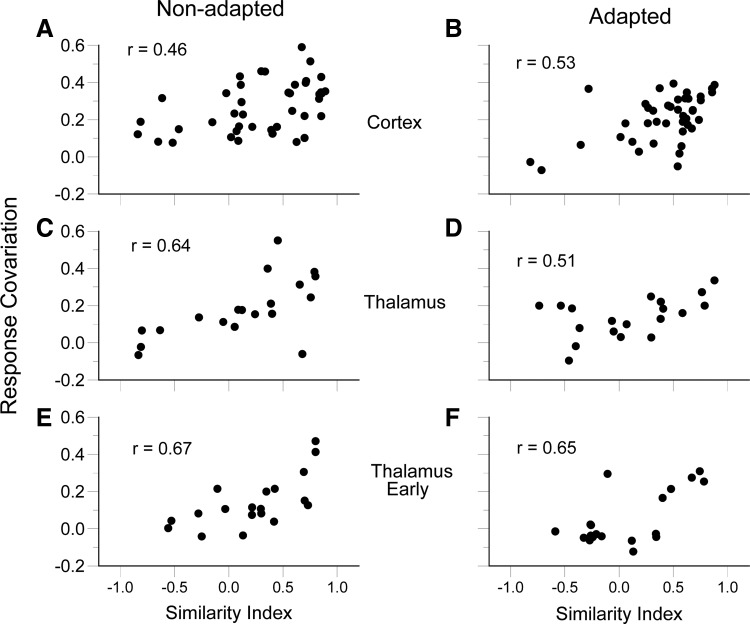
For central neurons, angular tuning similarity was a good predictor of firing covariation, but the relationship was considerably less robust than that among trigeminal ganglion cells. This is evident in the pair of simultaneously recorded TCUs whose trial-by-trial responses and trial-averaged polar plots are plotted in Fig. 3B. Although both units have similar angular tuning (SI = 0.80), compared with the trigeminal ganglion cells (Fig. 3A), their trial-by-trial responses are more variable with respect to deflection angle and with respect to one another (RC = 0.36). Population data for cortical pairs and thalamic pairs are plotted in Fig. 5. Pearson correlation coefficients for cortical (Fig. 5A) and thalamic (Fig. 5C) neurons are 0.46 and 0.64, with slopes of 0.13 ± 0.04 and 0.19 ± 0.05, respectively. The relationship between RC and SI is less robust in thalamus and cortex relative to primary afferent neurons (χ2 test for correlation coefficients, P values ≪0.001). Correlation coefficients for the two groups of central neurons are statistically equivalent as are their slopes (P values >0.05). Similar results for RC versus SI were obtained when only the early part of the thalamic response was examined (Fig. 5E). As in the case of the trigeminal ganglion cell analyses, shuffling the order of stimulus presentations leads to thalamic and cortical RC versus SI correlations of approximately 0.0. Thus within the whisker-to-barrel pathway, response covariation is stimulus specific, but the relationship is less robust centrally than predicted by stimulus-specific effects alone.
FIG. 5.
SI vs. RC. The relationship between angular tuning SI and trial-by-trial RC for nonadapted simultaneously recorded neuron pairs: cortex time window 5–25 ms after stimulus onset (A); thalamus time window 4–24 ms after stimulus onset (C); early thalamic response time window 4–9 ms after stimulus onset (E). Note that these relationships are significant (P < 0.01), but weaker than for pairs of trigeminal ganglion pairs. The stimulus dependence of response covariation is maintained after adaptation: barrel (B), thalamus (D), early thalamic response (F).
A possible explanation for the central decrease in stimulus-specific response covariation is that the responses of individual thalamic and cortical recordings are smaller and less variable. However, this was not the case. The average response magnitudes, calculated as spikes per stimulus over all eight deflections angles, were trigeminal ganglion, 0.70 ± 0.11; thalamus, 1.92 ± 0.14; and cortex, 1.68 ± 0.12. The coefficients of variation were highly similar for all three groups of neurons (trigeminal ganglion = 0.51; thalamus = 0.45; cortex = 0.55). Thus the observed central decrease in stimulus-specific response covariation does not solely reflect greater response variability in thalamic and cortical neurons.
Response covariation and stimulus-nonspecific effects
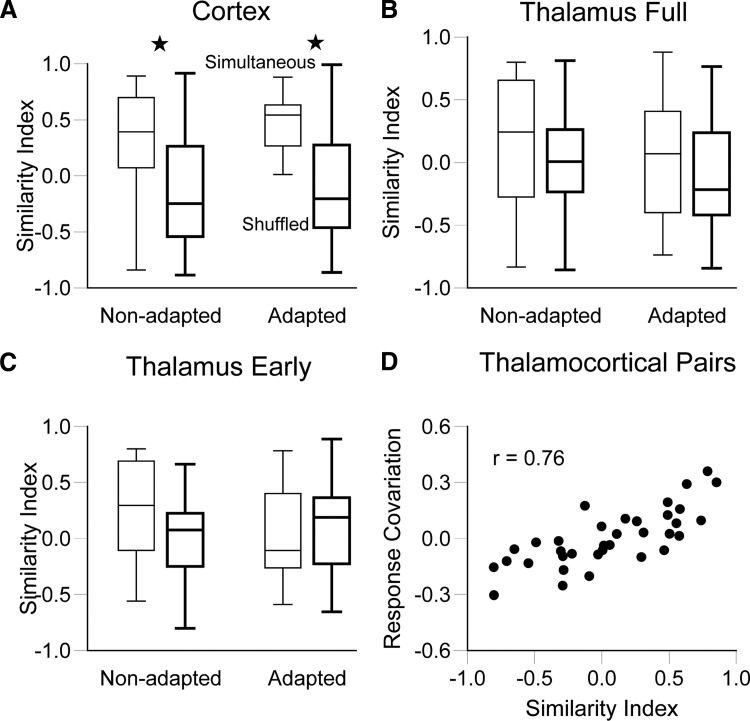
In both cortex and thalamus, most RC values are >0, whereas they are uniformly distributed from −1.0 to 1.0 in the trigeminal ganglion (see Fig. 4 and the following text). This finding suggests that networkwide fluctuations of background neural activity substantially influence whether individual neurons fire similarly or dissimilarly during any particular stimulus presentation. For example, in some trials both units may be more likely to respond to a stimulus due to spontaneous, common increases in network excitability, whereas during other trials, both units may be further from their response thresholds. We therefore predicted that trial-averaged angular tuning (i.e., polar plots) should be more similar for simultaneously recorded units than for units recorded at different times. To evaluate this prediction, we compared data obtained during simultaneous recordings to data obtained from randomly paired, nonsimultaneously recorded cells (see methods). For barrel neurons (Fig. 6A), SI values in the nonadapted condition were significantly higher for simultaneously recorded units than SI values calculated from 41 shuffled pairs (0.29 ± 0.08 vs. −0.08 ± 0.08; P = 0.0024). We conclude that a common source of input strongly determines whether barrel cells respond together irrespective of deflection angle. This accounts for the skew in the data points toward high SI values in Fig. 5, A and B.
FIG. 6.
Characteristics of simultaneously recorded thalamic and cortical neurons. Comparison of SIs between simultaneously recorded and shuffled neuron pairs, pre- and postadaptation, for cortical barrel (A), thalamus (B), and early thalamic (C) responses. In both preadaptation barrel pairs and early thalamic responses, the SIs of simultaneously recorded neurons were greater than those of shuffled pairs (P < 0.01). However, after adaptation, only the barrel pairs display greater than chance SI values postadaptation. In D, RC is plotted as a function of SI for unconnected thalamocortical pairs.
To determine whether thalamic inputs are responsible for the greater tuning similarity among simultaneously recorded versus separately recorded cortical units, we performed two additional analyses on thalamic responses. First, as before, we compared SIs of simultaneously recorded and shuffled thalamic units. Unlike the cortical findings, the full responses of simultaneously recorded thalamic units were as dissimilarly tuned for deflection angle as were 19 randomly shuffled pairs (simultaneous = 0.12 ± 0.13 vs. shuffled = 0.05 ± 0.09, P = 0.41) (Fig. 6B). For early thalamic responses as well, SI values were similar to those for random pairs (simultaneous = 0.23 ± 0.10 vs. shuffled = 0.08 ± 0.09, P = 0.33) (Fig. 6C).
In a second analysis, we examined response covariation within the thalamocortical circuit by analyzing data from simultaneously recorded single units in thalamic barreloid and cortical barrel neurons; data were taken from a previous study using similar recording and whisker stimulation conditions (Bruno and Simons 2002), except that no adaptation protocol was used. Here we examined 41 of the topographically aligned cell pairs that were deemed not to be synaptically connected on the basis of cross-correlation analyses; unconnected pairs were analyzed because Bruno and Simons (2002) showed that connected thalamic and cortical neurons are likely to have similar angular tuning. If the cortex receives a nonspecific, synchronizing influence from the thalamus, then even unconnected barreloid and barrel neurons should display RC values >0. This was not the case, however, because the mean RC value for unconnected thalamocortical pairs was 0.01 ± 0.03. This low mean RC value is not due to a bias wherein the paired thalamic and cortical neurons have a preponderance of dissimilarly tuned pairs because SI values vary from −1.0 to 1.0 (Fig. 6D). These two analyses combined thus suggest that the large stimulus-nonspecific component of response covariation among cortical neurons likely reflects a strong contribution of intracortical mechanisms.
We examined spontaneous firing by computing RC values for a 20-ms period just preceding stimulus onset. Median values for both populations were slightly greater than zero (cortex: 0.07; thalamus: 0.04). Spontaneous RC values for cortical and thalamic neurons did not differ (P = 0.13); however, for cortical but not thalamic pairs, prestimulus RC and polar plot similarity indices based on the subsequent whisker-evoked deflection were well correlated (cortex: r = 0.34, P = 0.03; thalamus: r = 0.26, P = 0.29). More similarly tuned barrel neurons thus appear to be more strongly influenced by common local fluctuations in background activity. Perhaps, such cells are located in the same or different but similarly tuned angular tuning domains (Bruno et al. 2003).
Effects of adaptation
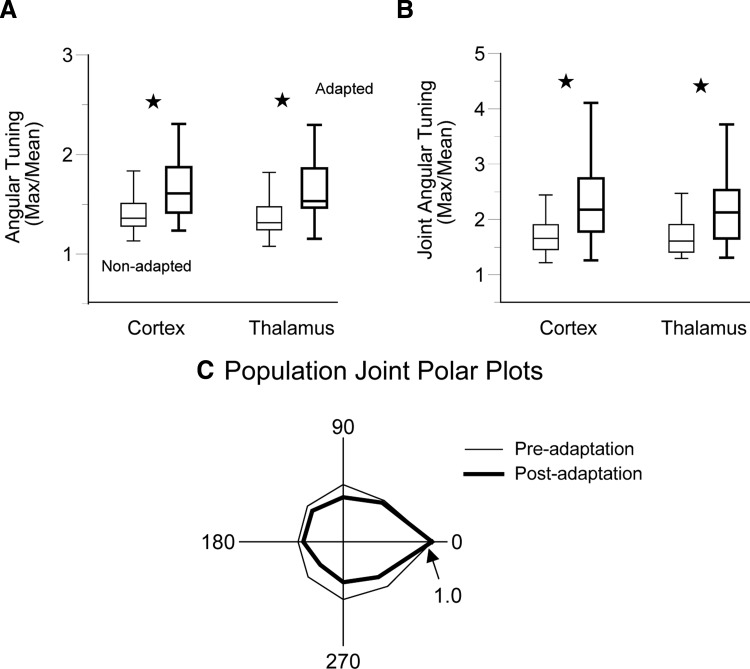
We previously found that sensory adaptation with prior whisker deflections enhances angular tuning in individual thalamic and cortical neurons (Khatri and Simons 2007). Here, we examined whether adaptation influences the joint firing of barrel pairs and thalamic pairs. We examined whether adaptation sharpens the joint tuning of simultaneously recorded neurons. In other words, does sensory adaptation make the joint spiking behavior of neurons more dependent on the particular direction of a whisker deflection? Stimulus-specific joint firing may enhance the saliency of afferent signals in feedforward excitatory circuits (see Feldmeyer et al. 2002; Usrey et al. 2000). The same ramp-and-hold stimuli as before were delivered to the whisker, but each was now preceded by 2.5 cycles of a 20-Hz adapting stimulus that terminated 25 ms prior to the ramp's onset (see Fig. 1). We compared on responses evoked by the pre- and postadaptation ramps. We observed close to 40% reductions in response magnitudes as a result of the adapting stimulus. As also reported previously (Khatri and Simons 2007), adaptation sharpens angular tuning in both thalamic and cortical neurons (P values <0.01, Fig. 7A). Here, we find that adaptation additionally increases trial-by-trial joint angular tuning of simultaneously recorded neurons (Fig. 7B). For thalamic and cortical recordings, postadaptation joint angular tuning indices (see methods) were larger than preadaptation indices (thalamus: 2.18 ± 0.15 vs. 1.73 ± 0.11, P < 0.001; cortex: 2.45 ± 0.17 vs. 1.86 ± 0.13, P ≪ 0.001). The enhanced postadaptation joint angular tuning is illustrated by population joint tuning polar plots (Fig. 7C). Neuron pairs showing the largest increases in joint angular tuning were not necessarily the same pairs that were jointly well tuned prior to adaptation or that showed the greatest adaptation decreases in response magnitudes (Pearson correlation coefficient, P > 0.11).
FIG. 7.
Adaptation and angular tuning. A: for individual units, adaptation sharpens angular tuning in both the thalamus and the cortex (P values ≪0.001). B: joint angular tuning is also enhanced by adaptation (P values <0.001). C: population joint tuning polar plots also depict sharper angular tuning. The population polar plot was constructed after rotating each individual polar plot such that the maximal response angle was set to zero and its responses were normalized to the maximal response.
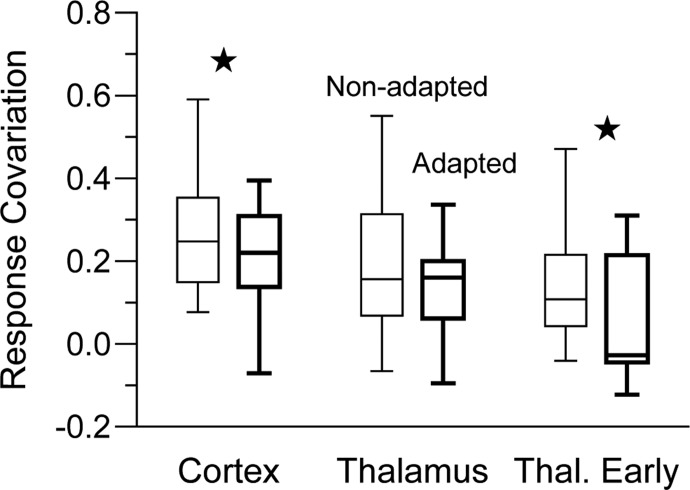
In addition to sharpening joint angular tuning, adaptation was associated with less response covariation (Fig. 8). In the cortex, adaptation reduced overall RC values around 20%, from a mean of 0.27 ± 0.02 (median = 0.25) to 0.21 ± 0.02 (median = 0.22) (Wilcoxon's signed-ranks test, P = 0.004). In thalamic units, covariation of responses measured over the full-response window was unaffected by adaptation (pre vs. post: 0.18 ± 0.04, median = 0.16 vs. 0.14 ± 0.03, median = 0.16, P = 0.21). However, firing during the first 5 ms of the thalamic response becomes decorrelated, as indicated by smaller postadaptation RC values (pre vs. post: 0.14 ± 0.03, median = 0.12 vs. 0.05 ± 0.03, median = 0.05, P = 0.014). We examined whether the amount of adaptation influences RC by correlating RC with a measure of joint adaptation calculated as the product of the evoked rates of both units in a pair. In neither the cortex nor the thalamus was there a significant relationship (P values >0.25).
FIG. 8.
Effects of adaptation on RC. Pre- and postadaptation RC values for cortical, thalamus, and early thalamus responses. Adaptation reduces RC in barrel neurons (P = 0.004) and the early thalamic response (P = 0.014), but not the full thalamic response.
Despite adaptation-induced reductions of response covariation in thalamus and cortex, the influence of the whisker stimulus remained robust, as indicated by strong correlations between SI and RC (cortex: r = 0.53, slope = 0.17 ± 0.04, P ≪ 0.001; thalamus full response: r = 0.51, slope = 0.11 ± 0.05, P = 0.003; thalamus early response: r = 0.65, slope = 0.23 ± 0.05, P = 0.003; see Fig. 5, B, D, and F). In addition, after adaptation the mean SI of simultaneously recorded barrel neurons (0.41 ± 0.06) was still significantly larger than shuffled values (P ≪ 0.001), indicating that stimulus-independent effects remained robust in the cortex despite decorrelating effects of adaptation. Thus like the nonadapted state, in the adapted state, response properties are a product of both stimulus-specific and stimulus-nonspecific effects.
DISCUSSION
The present study in the whisker-to-barrel system examined the degree to which stimulus-evoked neuronal firing in thalamocortical circuits is stimulus specific and stimulus nonspecific. Previous studies in awake primates have shown that adaptation decorrelates the activity of awake primary visual cortex neurons (Gutnisky and Dragoi 2008) and that somatosensory cortical neurons display stimulus-evoked firing covariation that is independent of the specific characteristics of the sensory stimulus (Romo et al. 2003). The present study, conducted in lightly sedated rats, extends these findings by demonstrating that stimulus-nonspecific effects are present in the thalamus and that decorrelation observed in the cortex could originate in the thalamus. The latter findings are consistent with cross-correlation analyses showing that firing synchrony among pairs of thalamic barreloid neurons diminishes rapidly during sensory adaptation produced by repetitive whisker deflection (Temereanca et al. 2008). Nevertheless, present data indicate that synchronizing effects are stronger in the cortex and present even within sensory-adapted cortical networks.
Angular tuning served as a probe for stimulus specificity because this is a prominent property of vibrissa-activated neurons throughout the whisker-to-barrel pathway (e.g., Shipley 1974; Simons and Carvell 1989; Zucker and Welker 1969). Our analyses indicate that trigeminal ganglion cells, which are highly sensitive to details of a whisker's deflection (Jones et al. 2004; Lichtenstein et al. 1990; Shoykhet et al. 2000), fire conjointly or fail to fire conjointly in a fashion that is strongly dependent on the direction of the stimulus and each cell's angular tuning. Stimulus-evoked conjoint firing of thalamic barreloid and cortical barrel neurons is less strongly determined by the specific parameters of the stimulus, i.e., deflection angle. Strikingly, covariation in trial-by-trial responses of simultaneously recorded cells leads to greater than expected similarities in trial-averaged angular tuning; that is, arbitrarily selected cells within a barrel are likely to display similar response properties, provided they are recorded at the same time. Similarly, simultaneously recorded cells in primate visual cortex are biased toward having similar receptive field properties (Kohn and Smith 2005).
Compared with primary afferent neurons, the degree of stimulus-evoked correlated firing in central neurons is less dependent on the characteristics of the stimulus itself (e.g., deflection angle). These findings are consistent with the view that, at any given moment, the response of an individual central neuron is a product of the interaction between the stimulus and the background state of thalamic and cortical networks. Our results in cortical barrels parallel the work of Arieli et al. (1996) in cat cortical area 17, which revealed that only 20% of a neuron's response is determined by the stimulus itself; the other 80% is due to the background state of the cortex. Strong trial-by-trial covariation in responsiveness is observed also in layer 2/3 cells of the barrel cortex of anesthetized mice (Sato et al. 2007). An interesting, unresolved issue is whether all nearby cortical neurons are equivalently affected by synchronizing background activity or whether neurons in the same local microcircuit are more strongly synchronized with each other.
Response similarity among cortical neurons could reflect strong conjoint responses in the thalamic neurons that provide input to them. However, simultaneously recorded pairs of topographically aligned thalamic barreloid and cortical barrel neurons displayed little stimulus-nonspecific response covariation. Thus stimulus-nonspecific response covariation among cortical neurons does not appear to reflect solely strong synchronization within the thalamus or within the recurrently connected thalamo-cortical-thalamic loop.
Mechanisms underlying response covariation
Response covariation between pairs of trigeminal ganglion cells and thalamic and/or cortical neurons was strongly related to the degree of similarity of the cells' angular tuning. Low-threshold primary afferent neurons respond to whisker deflections robustly and with little trial-to-trial variability (Jones et al. 2004) and there are no synaptic interactions among the cells. Stimulus-evoked firing among trigeminal neurons is conveyed centrally by angularly specific, feedforward excitatory projections at each synaptic station in the whisker-to-barrel pathway (Bruno et al. 2003; Minnery et al. 2003). In addition to the coordinating effects of this afferent activity, mechanisms within thalamic and cortical circuitry strongly determine the degree to which neurons respond similarly—or fail to respond at all—to the occurrence of a peripheral stimulus. Effects, though observed both within thalamic barreloids and cortical barrels, are likely mediated by different factors reflective of differences in the local circuitry. Cortical barrels are characterized by extensive intrabarrel synaptic interconnections between and among excitatory and inhibitory cells and these, in turn, are coupled to neurons elsewhere in the barrel cortex via intracolumnar and then intercolumnar projections (Armstrong-James 1975; Egger et al. 2008; Feldmeyer et al. 2005; Laaris et al. 2000; Lübke et al. 2003; Simons 1978). Such interconnectedness provides a substrate for spatially widespread spontaneous fluctuations in the excitability of cortical neurons that are present even in the awake state (Leopold et al. 2003). Thalamic barreloid neurons, on the other hand, do not synapse on one another and interact only indirectly, via feedback from inhibitory neurons in the thalamic reticular nucleus and from excitatory corticothalamic neurons in the infragranular layers of the barrel cortex. Under some conditions, both can synchronize thalamic firing (e.g., Andolina et al. 2007; Destexhe et al. 1998). Nevertheless, the high degree of direct coupling among cortical barrel neurons relative to the more indirect nature of interactions among thalamic barreloid neurons is consistent with our findings that cortical responses are less dependent on feedforward, excitatory stimulus-specific inputs.
Direct effects of a given sensory stimulus can also be diminished as a result of synaptic convergence and divergence, especially if a given cell receives weak inputs from numerous presynaptic neurons having slightly different stimulus-specific, i.e., angular tuning, properties. Such an architecture is a prominent feature of the barreloid-to-barrel projection, where a single excitatory barrel neuron receives relatively weak inputs from as many as 90 thalamocortical neurons (Bruno and Sakmann 2006; Bruno and Simons 2002). A given thalamic barreloid neuron, on the other hand, receives highly efficacious synaptic inputs from only a few neurons in the brain stem principal sensory nucleus (Deschênes et al. 2003) and it appears that each brain stem neuron, in turn, receives strong inputs from only a limited number of trigeminal ganglion cells (Minnery and Simons 2003; Minnery et al. 2003). The stronger, more spatially tuned synaptic inputs onto thalamic neurons would render responses of barreloid neurons more tightly coupled to afferent activity than cortical neurons, as observed in the present study.
Response covariation and sensory adaptation
In the whisker-to-barrel system, adaptation within thalamocortical circuits leads to a reduction in already sparse firing (Ahissar et al. 2000; Castro-Alamancos 2004; Garabedian et al. 2003; Khatri and Simons 2007; Khatri et al. 2004). Diminished response magnitudes likely reflect a combination of synaptic depression of excitatory synapses and locally generated inhibition. Neuromodulators, such as acetylcholine, may also contribute to lower firing rates, especially during alert wakefulness (Fournier et al. 2004). In the present study we observed that sensory adaptation induced by prior whisker deflections diminishes response covariation. Recent theoretical work (de la Rocha et al. 2007) demonstrates that correlations between neural spike trains are strongly dependent on both the coupling strength between neurons and their joint firing rate. In the de la Rocha study, for a given degree of synaptic coupling (unknown in our recordings), firing correlation decreases with lower joint firing rates, the exact relationship being determined by nonlinear properties of the neurons. These findings suggest that the adaptation-induced reduction in overall response covariation observed here is a consequence of diminished spiking activity rather than the recruitment of mechanisms that might function to oppose firing synchrony, such as fractionation of the barrel into out-of-phase microcircuits.
With adaptation, whisker-evoked responses, although reduced in magnitude, display sharpened angular tuning, smaller receptive fields, and more temporally focused firing (Castro-Alamancos 2004; Khatri and Simons 2007; Khatri et al. 2004). Present findings show additionally that adaptation enhances the conjoint angular tuning of simultaneously recorded neurons. This is likely due to combined effects of elevated firing thresholds, which enhances angular tuning, and aforementioned circuit-level mechanisms of neuronal synchronization, which operate in the adapted state, albeit at reduced levels. Adaptation leads to an overall decrease in response covariation. Enhanced joint angular tuning within a context of general decorrelation indicates that in the adapted state responses of any two cells to nonpreferred or ineffective stimuli are more likely to vary independently of each other.
Sensory adaptation decorrelates barrel neuron firing, even among neurons having initially similar angular preferences. Indeed, on average, our measure of firing similarity decreased in nearly 60% of simultaneously recorded pairs of barrel neurons. The decorrelating effect appears to be general, in that decreases in response covariation were as likely to occur when cells had the same preadaptation angular tuning as when they did not. It remains unclear whether the subset of cell pairs displaying adaptation-induced decreases in response covariation differ with respect to other characteristics of potential functional significance, such as location in different angular tuning domains (Bruno et al. 2003) or in different subbarrels (Land and Erickson 2005). Nevertheless, even after adaptation, response covariation is still present for neurons that are similarly tuned and likely to be influencing the same postsynaptic targets. Taken against a background of overall lower firing rates, response decorrelation, and stronger joint angular tuning, these coordinated neuronal groups are likely to have a particularly substantial impact on postsynaptic cells, e.g., in supragranular layers, during alert wakefulness, when thalamocortical circuits are strongly adapted (Castro-Alamancos 2004) and trial-averaging is not available for sensory discrimination.
GRANTS
This work was supported by National Institute of Neurological Disorders and Stroke Grant NS-19950.
Acknowledgments
We thank H. T. Kyriazi and A. Su for assistance with histological processing and surgical preparations.
Present address of R. M. Bruno: Department of Neuroscience, Columbia University, New York, New York.
REFERENCES
- Ahissar 2000.Ahissar E, Sosnik R, Haidarliu S. Transformation from temporal to rate code coding in a somatosensory thalamocortical pathway. Nature 406: 302–306, 2000. [DOI] [PubMed] [Google Scholar]
- Andolina 2007.Andolina IM, Jones HE, Wang W, Sillito A. Corticothalamic feedback enhances stimulus response precision in the visual system. Proc Natl Acad Sci USA 104: 1685–1690, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arieli 1996.Arieli A, Sterkin A, Grinvald A, Aertsen A. Dynamics of ongoing activity: explanation of the large variability in evoked cortical responses. Science 273: 1868–1871, 1996. [DOI] [PubMed] [Google Scholar]
- Armstrong-James 1975.Armstrong-James M The functional status and columnar organization of single cells responding to cutaneous stimulation in neonatal rat somatosensory cortex S1. J Physiol 246: 501–538, 1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlow 1989.Barlow HB, Földiák P. Adaptation and decorrelation in the cortex. In: The Computing Neuron, edited by Durbin RM, Miall C, Mitchison GJ. Boston, MA: Addison–Wesley Longman Publishing, 1989, p. 54–72.
- Bruno 2003.Bruno RM, Khatri V, Land PW, Simons DJ. Thalamocortical angular tuning domains within individual barrels of rat somatosensory cortex. J Neurosci 23: 9565–9574, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruno 2006.Bruno RM, Sakmann B. Cortex is driven by weak but synchronously active thalamocortical synapses. Science 312: 1622–1627, 2006. [DOI] [PubMed] [Google Scholar]
- Bruno 2002.Bruno RM, Simons DJ. Feedforward mechanisms of excitatory and inhibitory cortical receptive fields. J Neurosci 22: 10966–10975, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro-Alamancos 2004.Castro-Alamancos MA Absence of rapid sensory adaptation in neocortex during information processing states. Neuron 41: 455–464, 2004. [DOI] [PubMed] [Google Scholar]
- de la Rocha 2007.de la Rocha J, Doiron B, Shea-Brown E, Josic K, Reyes A. Correlation between neural spike trains increases with firing rate. Nature 448: 802–806, 2007. [DOI] [PubMed] [Google Scholar]
- Deschênes 2003.Deschênes M, Timofeeva E, Lavallée P. The relay of high-frequency sensory signals in the whisker-to-barreloid pathway. J Neurosci 23: 6778–6787, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Destexhe 2006.Destexhe A, Contreras D. Neuronal computations with stochastic network states. Science 314: 85–90, 2006. [DOI] [PubMed] [Google Scholar]
- Destexhe 1998.Destexhe A, Contreras D, Steriade M. Mechanisms underlying the synchronizing action of corticothalamic feedback through inhibition of thalamic relay cells. J Neurophysiol 79: 999–1016, 1998. [DOI] [PubMed] [Google Scholar]
- DeWeese 2005.DeWeese MR, Hromádka T, Zador AM. Reliability and representational bandwidth in the auditory cortex. Neuron 48: 479–488, 2005. [DOI] [PubMed] [Google Scholar]
- Diesmann 1999.Diesmann M, Gewaltig MO, Aersten A. Stable propagation of synchronous spiking in cortical neural networks. Nature 402: 529–533, 1999. [DOI] [PubMed] [Google Scholar]
- Egger 2008.Egger V, Nevian T, Bruno RM. Subcolumnar dendritic and axonal organization of spiny stellate and star pyramid neurons within a barrel in rat somatosensory cortex. Cereb Cortex 18: 876–889, 2008. [DOI] [PubMed] [Google Scholar]
- Feldmeyer 2002.Feldmeyer D, Lübke J, Silver RA, Sakmann B. Synaptic connections between layer 4 spiny neurone-layer 2/3 pyramidal cell pairs in juvenile rat barrel cortex: physiology and anatomy of interlaminar signaling within a cortical column. J Physiol 538: 803–822, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldmeyer 2005.Feldmeyer D, Roth A, Sakmann B. Monosynaptic connections between pairs of spiny stellate cells in layer 4 and pyramidal cells in layer 5A indicate that lemniscal and paralemniscal afferent pathways converge in the infragranular somatosensory cortex. J Neurosci 25: 3423–3431, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fournier 2004.Fournier GN, Semba K, Rasmusson DD. Modality and region-specific acetylcholine release in the rat neocortex. Neuroscience 126: 257–262, 2004. [DOI] [PubMed] [Google Scholar]
- Fraser 2006.Fraser G, Hartings JA, Simons DJ. Adaptation of trigeminal ganglion cells to periodic whisker deflections. Somatosens Mot Res 23: 111–118, 2006. [DOI] [PubMed] [Google Scholar]
- Garabedian 2003.Garabedian CE, Jones SR, Merzenich MM, Dale A, Moore CI. Band-pass response properties of rat SI neurons. J Neurophysiol 90: 1379–1391, 2003. [DOI] [PubMed] [Google Scholar]
- Gutnisky 2008.Gutnisky DA, Dragoi V. Adaptive coding of visual information in neural populations. Nature 452: 220–224, 2008. [DOI] [PubMed] [Google Scholar]
- Hartings 2003.Hartings JA, Temereanca S, Simons DJ. State-dependent processing of sensory stimuli by thalamic reticular neurons. J Neurosci 23: 5264–5271, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasenstaub 2007.Hasenstaub A, Sachdev RN, McCormick DA. State changes rapidly modulate cortical neuronal responsiveness. J Neurosci 27: 9607–9622, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haslinger 2006.Haslinger R, Ulbert I, Moore CI, Brown EN, Devor A. Analysis of LFP phase predicts sensory responses in barrel cortex. J Neurophysiol 96: 1658–1663, 2006. [DOI] [PubMed] [Google Scholar]
- Jones 2004.Jones LM, Depireux DA, Simons DJ, Keller A. Robust temporal coding in the trigeminal system. Science 304: 1986–1989, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khatri 2004.Khatri V, Hartings JA, Simons DJ. Adaptation in thalamic barreloid and cortical barrel neurons to periodic whisker deflections varying in frequency and velocity. J Neurophysiol 92: 3244–3254, 2004. [DOI] [PubMed] [Google Scholar]
- Khatri 2007.Khatri V, Simons DJ. Angularly nonspecific response suppression in rat barrel cortex. Cereb Cortex 17: 599–609, 2007. [DOI] [PubMed] [Google Scholar]
- Kohn 2005.Kohn A, Smith MA. Stimulus dependence of neuronal correlation in primary visual cortex of the macaque. J Neurosci 25: 3661–3673, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumbhani 2007.Kumbhani RD, Nolt MJ, Palmer LA. Precision, reliability, and information-theoretic analysis of visual thalamocortical neurons. J Neurophysiol 98: 2647–2663, 2007. [DOI] [PubMed] [Google Scholar]
- Kyriazi 1998.Kyriazi H, Carvell GE, Brumberg JC, Simons DJ. Laminar differences in bicuculline methiodide's effects on cortical neurons in the rat whisker/barrel system. Somatosens Mot Res 15: 146–156, 1998. [DOI] [PubMed] [Google Scholar]
- Laaris 2000.Laaris N, Carlson GC, Keller A. Thalamic-evoked synaptic interactions in barrel cortex revealed by optical imaging. J Neurosci 20: 1529–1537, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Land 2005.Land PW, Erickson SL. Subbarrel domains in rat somatosensory (S1) cortex. J Comp Neurol 490: 414–426, 2005. [DOI] [PubMed] [Google Scholar]
- Land 1985.Land PW, Simons DJ. Cytochrome oxidase staining in the rat SmI barrel cortex. J Comp Neurol 238: 225–235, 1985. [DOI] [PubMed] [Google Scholar]
- Leopold 2003.Leopold DA, Murayama Y, Logothetis NK. Very slow fluctuations in monkey visual cortex: implications for functional brain imaging. Cereb Cortex 13: 422–433, 2003. [DOI] [PubMed] [Google Scholar]
- Lichtenstein 1990.Lichtenstein SH, Carvell GE, Simons DJ. Responses of rat trigeminal ganglion neurons to movements of vibrissae in different directions. Somatosens Mot Res 7: 47–65, 1990. [DOI] [PubMed] [Google Scholar]
- Lübke 2003.Lübke J, Roth A, Feldmeyer D, Sakmann B. Morphometric analysis of the columnar innervation domain of neurons connecting layer 4 and layer 2/3 of juvenile rat barrel cortex. Cereb Cortex 13: 1051–1063, 2003. [DOI] [PubMed] [Google Scholar]
- Minnery 2003.Minnery BS, Bruno RM, Simons DJ. Response transformation and receptive-field synthesis in the lemniscal trigeminothalamic circuit. J Neurophysiol 90: 1556–1570, 2003. [DOI] [PubMed] [Google Scholar]
- Minnery 2003.Minnery BS, Simons DJ. Response properties of whisker-associated trigeminothalamic neurons in rat nucleus principalis. J Neurophysiol 89: 40–56, 2003. [DOI] [PubMed] [Google Scholar]
- Mittmann 2005.Mittmann W, Koch U, Hausser M. Feed-forward inhibition shapes the spike output of cerebellar Purkinje cells. J Physiol 563: 369–378, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen 2003.Petersen CC, Hahn TT, Mehta M, Grinvald A, Sakmann B. Interaction of sensory responses with spontaneous depolarization in layer 2/3 neurons. Proc Natl Acad Sci USA 100: 13638–13643, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto 2000.Pinto DJ, Brumberg JC, Simons DJ. Circuit dynamics and coding strategies in rodent somatosensory cortex. J Neurophysiol 83: 1158–1166, 2000. [DOI] [PubMed] [Google Scholar]
- Pouille 2001.Pouille F, Scanziani M. Enforcement of temporal fidelity in pyramidal cells by somatic feed-forward inhibition. Science 293: 1159–1163, 2001. [DOI] [PubMed] [Google Scholar]
- Romo 2003.Romo R, Hernandez A, Zainos A, Salinas E. Correlated neuronal discharges that increase coding efficiency during perceptual discrimination. Neuron 38: 649–657, 2003. [DOI] [PubMed] [Google Scholar]
- Samonds 2003.Samonds JM, Allison JD, Brown HA, Bonds AB. Cooperation between area 17 neuron pairs enhances fine discrimination of orientation. J Neurosci 23: 2416–2525, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato 2007.Sato R, Gray NW, Mainen ZF, Svoboda K. The functional microarchitecture of the mouse barrel cortex. PLoS Biol 5: 1440–1452, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons 1978.Simons DJ Response properties of vibrissa units in rat SI somatosensory cortex. J Neurophysiol 41: 798–820, 1978. [DOI] [PubMed] [Google Scholar]
- Simons 1989.Simons DJ, Carvell GE. Thalamocortical response transformations in the rat vibrissa/barrel system. J Neurophysiol 61: 311–330, 1989. [DOI] [PubMed] [Google Scholar]
- Simons 1992.Simons DJ, Carvell GE, Hershey AE, Bryant DP. Responses of barrel cortex neurons in awake rats and effects of urethane anesthesia. Exp Brain Res 91: 259–272, 1992. [DOI] [PubMed] [Google Scholar]
- Swadlow 2001.Swadlow HA, Gusev AG. The impact of bursting thalamic impulses at a neocortical synapse. Nat Neurosci 4: 402–408, 2001. [DOI] [PubMed] [Google Scholar]
- Temereanca 2003.Temereanca S Thalamic Signal Processing and the Effects of Cortical Feedback (PhD thesis). Pittsburgh, PA: Univ. of Pittsburgh, 2003.
- Temereanca 2000.Temereanca S, Brown EN, Simons DJ. Rapid changes in thalamic firing synchrony during repetitive whisker stimulation. J Neurosci 28: 11153–11164. [DOI] [PMC free article] [PubMed]
- Usrey 2000.Usrey WM, Alonso JM, Reid RC. Synaptic interactions between thalamic inputs to simple cells in cat visual cortex. J Neurosci 15: 5461–5467, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zucker 1969.Zucker E, Welker WI. Coding of somatic sensory input by vibrissae neurons in the rat's trigeminal ganglion. Brain Res 12: 138–156, 1969. [DOI] [PubMed] [Google Scholar]