Abstract
The insertion of a heterologous gene into commensal bacteria is a common technique to develop a delivery agent for vaccination and therapies, but the pleiotropic effects of genetic modifications need to be investigated before its use in practical applications. Although supplemental properties provided by the expression of heterologous antigens have been reported, the negative or side effects on the immune-modulating properties caused by recombination are barely understood. In the present study, we fortuitously found that the secretion of tumor necrosis factor alpha (TNF-α) from murine macrophages was reduced by recombinant Lactobacillus casei expressing Salmonella OmpC compared to the stimulation of TNF-α secretion by nonexpressing L. casei. This reduction could not be attributed to OmpC as a purified protein. The main component of the OmpC-expressing strain included in the attenuation of TNF-α release seemed to be the cell wall, which exhibited higher sensitivity against N-acetylmuramidase than that of nonexpressing strains. These results suggest that the recombinant strain expressing a specific heterologous antigen might be digested rapidly in macrophages and lose immune-stimulating capability at an early time point.
Many strains of lactobacilli and other lactic acid bacteria are safe symbiotic bacteria, which have been used for food fermentation or exist in our gastrointestinal tract commensally. Among their favorable health properties, the immune-modulating properties of lactobacilli have been investigated in many reports (11, 18, 25, 28). More particularly, several cell components of Lactobacillus strains, such as lipoteichoic acid and oligodeoxynucleotides, are known to elicit innate immune responses through Toll-like receptors 2 and 9 (24, 35). There is also evidence that lactobacilli can evoke immune responses associated with the nucleotide binding oligomerization domain-like receptor family that primarily recognizes microbial molecules of bacterial origin, such as muramyl dipeptide (10, 18). As a genetic factor of lactic acid bacteria, recent studies reported that a mutant of Lactobacillus plantarum NCIMB 8826 and Lactobacillus rhamnosus GG deficient in the d-alanylation of teichoic acid converted their capacity of immunomodulation (13, 29).
Recently, several studies demonstrated that genetically modified lactobacilli exhibited properties for the induction of supplemental immune responses in combination with heterologous proteins such as pathogenic antigens for vaccination, allergens for anti-allergic treatments, and other responses (2, 5, 6, 12, 14, 19, 21, 23, 27, 28, 30, 32, 33). Previously, it was also reported that recombinant Lactobacillus casei ATCC 393 expressing flagellin from Salmonella enterica serovar Enteritidis (SE) could induce protective immunity (17). In these studies, recombinant lactic acid bacteria showed additional immunological or physiological activities that the wild-type strains did not provide originally. On the other hand, the opposite case that a heterologous protein could negatively affect the functions of the host strain has scarcely been examined. Because the insertion of a heterologous gene into commensal bacteria appears to be a common technique to develop a delivery agent for vaccination and therapies, the pleiotropic effects of genetic modifications should be investigated before practical applications are put into therapeutic use. In the course of developing a recombinant vaccine based on lactobacilli, it was fortuitously found that heterologous protein-expression reduced an immunological property of the host bacteria.
In the present study, it was found that recombinant L. casei ATCC 393 expressing SE OmpC induced less tumor necrosis factor alpha (TNF-α) production by murine macrophage-like cells than a nonexpressing strain. OmpC refers to a major outer membrane porin of Salmonella, the function of which is the formation of a channel for the diffusion of nutrients and low-molecular-weight compounds across the outer membrane (26). OmpC is also known as a protective antigen for vaccination against Salmonella because OmpC-specific antibodies exert a bactericidal effect (15, 16, 20). In this context, the recombinant Lactobacillus producing OmpC was originally constructed to be applied for vaccination; however, the recombinant lactobacilli showed weaker immunogenic properties than the original strain. Interestingly, attenuation of the immunostimulating property of recombinant L. casei was not attributed directly to OmpC. We report here how OmpC expression reduced the TNF-α-inducing capacity of recombinant L. casei.
MATERIALS AND METHODS
Bacterial cells and culture conditions.
A frequently used plasmid-free strain, L. casei ATCC 393 (but probably different from the original strain of ATCC 393T), was used as a host strain for genetic modification (1). All recombinant L. casei strains were grown in de Mann-Rogosa-Sharpe (MRS) medium supplemented with 5 μg of erythromycin/ml at 37°C. For heterologous-protein expression, recombinant lactobacilli were incubated in Lactobacillus-carrying medium (LCM) containing 1% mannitol and erythromycin at 37°C (22). As a negative control for the current experiments, L. casei carrying pLPEmpty, which was constructed in a previous study, was used (17). Escherichia coli JM109 (TaKaRa, Tokyo, Japan) was used as the cloning host and grown in LB broth containing 100 μg of ampicillin/ml. For the expression of His6-tagged protein, E. coli M15 (Qiagen, Tokyo, Japan) was grown in LB broth supplemented with ampicillin, kanamycin (25 μg/ml), and 1 mM IPTG (isopropyl-β-d-thiogalactopyranoside) at 37°C. S. enterica SE strain #40 is a clinically isolated laboratory strain and was grown in LB medium (17).
Preparation of recombinant SE OmpC and anti-OmpC anbibodies.
Histidine-tagged OmpC was prepared using E. coli M15 and vector pQE31 according to the manufacturer's instructions (Qiagen). The gene fragment encoding the signal peptide-deficient SE OmpC (C terminus of 315 amino acids) was amplified from SE chromosomal DNA by PCR using a pair of primers, IGM424 (5′-CCC CGG ATC CGG AAA CGC AGG TTA ACG ATC A) and IGM425 (5′-GGG GCT CGA GGA ACT GGT AAA CCA GAC CCA). The DNA segments were digested with BamHI and XhoI, followed by insertion into the BamHI-SalI site of pQE31. After the induction of protein expression, His-tagged proteins were purified under denaturing conditions. The molecular mass and purity of the prepared proteins were confirmed by sodium dodecyl sulfate-10% polyacrylamide gel electrophoresis and Coomassie brilliant blue staining. The concentration of the recombinant protein was determined by the Bradford protein assay (Bio-Rad, Tokyo, Japan). A high concentration (>1 mg/ml) of purified protein solution in phosphate-buffered saline (PBS) supplemented with 4 M urea was stored at −20°C until use. Protein renaturing was performed by rapid dilution in PBS or RPMI 1640 medium (Sigma-Aldrich Japan, Tokyo, Japan). Contamination with lipopolysaccharide (LPS) in the protein solution was detected using Endospecy (Seikagaku Corp., Tokyo, Japan) in accordance with the manufacturer's instructions. In order to prepare anti-OmpC antibodies, BALB/c mice were immunized intraperitoneally four times with 10 μg of His-tagged OmpC/mouse at 3-week intervals. Freund complete adjuvant was used only for the first injection. The care and use of experimental animals complied with local animal welfare laws and guidelines. Serum was prepared, and the titer of anti-OmpC antibodies was determined by enzyme-linked immunosorbent assay as described previously (17). The affinity of the antibodies to SE OmpC was evaluated by immunoblot analysis against a total cell extract of SE. The SE total cell extract was prepared by simple resuspension and boiling in Laemmli sample buffer.
Construction of recombinant L. casei.
As an expression vector for the cell-surface anchoring of the heterologous antigens, pLP401::OmpC, was constructed from pLP401, which was developed by Pouwels et al. (31). The gene fragment encoding OmpC of SE was amplified by PCR using a pair of primers, IGM424 and IGM425. The DNA segments were digested with BamHI and XhoI, followed by insertion into the same restriction sites of pLP401. The resultant plasmid (pLP401::OmpC), the expression cassette for which consisted of the promoter plus the signal sequence of amy, ompC, and the anchor of prtP, was introduced into L. casei by electroporation as described previously (31). Expression of the heterologous antigen was confirmed by Western blot analysis. The recombinant L. casei were incubated in LCM for 8 h, and bacterial cells and culture supernatants were separated by centrifugation. For preparing a whole-cell extract, the bacterial cells were treated with 5 mg of lysozyme/ml and 20 U of mutanolysin/ml in Tris-Cl (pH 8.0) supplemented with 0.3 M sucrose at 37°C for 30 min. The enzyme-treated cells were washed, resuspended in water, and lysed in the same volume of 2× Laemmli sample buffer. The proteins were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis using a 10 to 20% gradient gel and then electrically blotted onto a polyvinylidene difluoride membrane (Millipore, Kanagawa, Japan). For the quantification of OmpC expressed by recombinant L. casei, the specific signals were detected using an Alexa Fluor 488-labeled antibody (Molecular Probes). The specific bands were analyzed using Molecular Imager FX and Quantity One (Bio-Rad). The amount of OmpC produced by the recombinant strain was estimated by comparing the density of the band to purified OmpC standards. The surface location of OmpC on the bacterial cell was analyzed by using a FACSCalibur instrument and CellQuest software (BD, Tokyo, Japan). The bacterial cells incubated in LCM were collected, washed, and suspended in PBS supplemented with 0.05% Tween 20 and 1% bovine serum albumin. The cells were incubated with anti-OmpC antibodies and Alexa Fluor 488-labeled anti-mouse immunoglobulin G (IgG; Molecular Probes). Ten thousand events were analyzed by using a flow cytometer.
Evaluation of cell viability of recombinant lactobacilli.
The cell viability of recombinant lactobacilli was determined by using a Live/Dead BacLight bacterial counting and viability kit (Molecular Probes). By staining with SYTO 9 and propidium iodide, “live” (intact cell membrane/wall) bacteria exhibit bright fluorescence, whereas “dead” (damaged cell membrane/wall) bacteria exhibit weak fluorescence. Logarithmically growing (8 h) cultures in LCM broth were stained with the two dyes and applied to flow cytometry. Ten thousand events were analyzed using a FACSCalibur instrument and plotted on a dot plot cytogram. The concentration of cell particles was calculated by comparison to microsphere standards. More detailed information is described in the manufacturer's protocol. Cell viability was also determined by the regular colony counting method. The CFU counts per cell particle were determined by using a flow cytometer. The percentage of colony-forming cells was calculated.
Preparation of bacterial cells and cell components.
Heat-killed recombinant L. casei cells were prepared from fresh cultures. Prewarmed LCM/mannitol broth was inoculated with an overnight culture of recombinant lactobacilli in MRS broth and incubated for 8 h at 37°C. Bacterial cells were then collected, washed with PBS and distilled water, and heat killed at 80°C for 20 min, followed by lyophilization. Fluorescein isothiocyanate (FITC)-labeled lactobacilli were prepared with FITC-I (Dojindo, Kumamoto, Japan) as described by Shida et al. (34). Briefly, heat-killed bacterial cells were incubated in 50 mM carbonate buffer (pH 9), including 4.5 μg of FITC-I/ml, at 37°C for 1 h and then washed with PBS. A cell wall-removed fraction (CWRF) of recombinant lactobacilli was prepared from the heat-killed cells. The cells were treated with lysozyme (10 mg/ml) and mutanolysin (20 U/ml) in 50 mM Tris-Cl buffer supplemented with 0.3 M sucrose at 37°C for 16 h and washed sufficiently with the same buffer. An intact cell wall (ICW) sample was prepared as described previously (24). In short, heat-killed cells were boiled in 0.3% sodium dodecyl sulfate solution and washed thoroughly. The cell pellets were then treated with pronase, followed by delipidation with methanol, methanol-chloroform-water, and methanol-chloroform. Nucleotides were digested with benzonase, and the remaining cell particles were washed thoroughly with distilled water.
Cell culture.
Murine peritoneal macrophages and the macrophagelike cell line RAW264.7 were maintained in RPMI 1640 medium supplemented with 10% fetal bovine serum and penicillin-streptomycin (complete medium) at 37°C in a 5% CO2 incubator. Murine peritoneal macrophages were isolated from female BALB/c mice (10-13 weeks old) that were injected intraperitoneally with 4% thioglycolate medium 3 days before sampling. Peritoneal lavage fluid in PBS was collected and washed with complete medium. The care and use of experimental animals complied with local animal welfare laws and guidelines. The murine macrophagelike cell line RAW264.7 was purchased from the American Type Culture Collection. Each cell suspension was seeded in a 96-well microplate (2 × 104 cells/well) and incubated for 3 h to promote cell attachment. Nonadherent cells were removed, and then fresh medium, including purified protein (1 ng/ml or 1 μg/ml), heat-killed bacterial cells (0.5 to 10 μg/ml), or bacterial components, was added. For some experiments, an antiendotoxin reagent, polymyxin B nonapeptide (Sigma), or purified E. coli O55:B5 LPS (Sigma) was added to the medium at 100 μg/ml (7). After 24 h of incubation (or at other time points as indicated), cleared culture supernatants were collected by centrifugation and stored at −20°C.
Cytokine quantification and cytotoxicity assay.
TNF-α and interleukin-10 (IL-10) released into the culture supernatants were detected with TNF-α and IL-10 OptEIA enzyme-linked immunosorbent assay sets (BD Biosciences, San Diego, CA), respectively. Appropriately diluted culture supernatants were assayed in accordance with the manufacturer's instructions. Concentrations of the cytokines were calculated using a standard curve. The cytotoxicity of killed bacteria during incubation with macrophages was determined with a CytoTox 96 nonradioactive cytotoxicity assay (Promega, Tokyo, Japan). In accordance with the manufacturer's protocol, free lactate dehydrogenase (LDH) leaked from the damaged cells was detected.
Flow cytometry analysis of macrophages.
Preparation of macrophages for flow cytometry analysis was performed according to the methods described by Shida et al. (34). RAW264.7 cells (105 cells in 24-well plates) were incubated with FITC-conjugated bacteria in complete medium. Macrophages were dislodged by treatment with PBS supplemented with 10 mM EDTA, washed with PBS/EDTA, and fixed in 1.25% formalin-PBS. The cells were then analyzed by using a FACSCalibur cytometer and CellQuest software.
N-Acetylmuramidase treatment.
Heat-killed lactobacilli were suspended in 50 mM Tris-Cl buffer (pH 8) supplemented with 10 μg of mutanolysin/ml and incubated for 0, 10, 30, 60, and 120 min. The enzyme-treated cells were lysed in 2% sodium dodecyl sulfate solution, and the optical density was measured at 600 nm (OD600).
Statistical analysis.
Statistical significance was evaluated by using Student t test and one-way analysis of variance. Significant differences were defined as a P value of <0.05.
RESULTS
Construction and evaluation of recombinant L. casei.
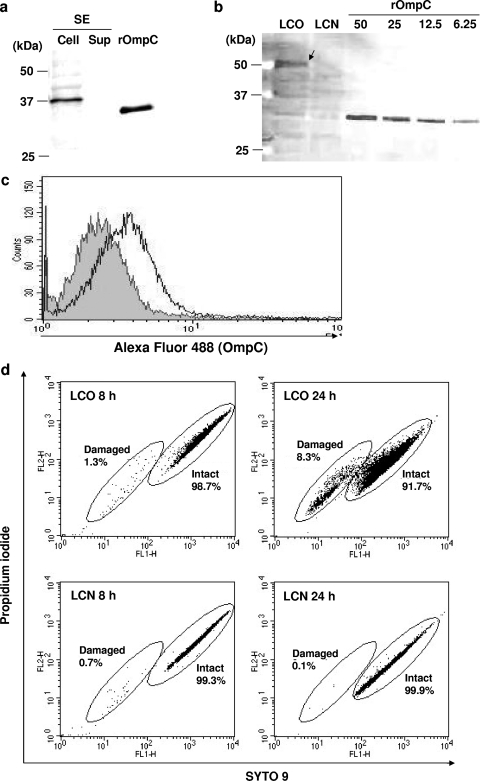
The expression of SE OmpC in recombinant L. casei was confirmed by immunoblotting. The specificity of anti-OmpC antibodies was validated by the detection of a specific appropriate-sized band in the cellular fraction of an SE culture (Fig. 1a). As shown in Fig. 1b, the OmpC-specific band of recombinant L. casei carrying pLP401::OmpC (LCO) was detected from its cell extract, while no specific band was detected from a control strain carrying pLPEmpty (LCN). The greater molecular mass of OmpC from LCO than purified recombinant OmpC (rOmpC) was appropriate because OmpC was fused to an anchor peptide provided from the pLP401 vector. The amount of heterologous antigen was also estimated by comparing to the signal intensity of purified recombinant OmpC. The expression efficiency of the antigen was approximately 25 ng per 2 × 108 CFU.
FIG. 1.
Construction of recombinant L. casei expressing OmpC. (a) Specific antibody for the detection of OmpC. Purified recombinant OmpC and anti-OmpC antibodies were analyzed by immunoblotting. Approximately 108 CFU of SE whole-cell extract (cell), the corresponding volume of culture supernatant (Sup), and 25 ng of purified OmpC (rOmpC) were applied. The sizes of the molecular mass markers are shown in the left margin. (b) Detection and quantification of OmpC expressed by recombinant L. casei. Whole-cell extracts of OmpC-expressing L. casei and a nonexpressing strain (corresponding to 2 × 108 CFU/lane) were applied to immunoblotting. The blot was conjugated with anti-OmpC antibody and Alexa Fluor 488-labeled IgG. An OmpC-specific band was visualized using Molecular Imager FX and analyzed with Quantity One (Bio-Rad). The sizes of the molecular mass markers are shown in the left margin. LCO, OmpC-expressing L. casei; LCN, nonexpressing L. casei. The values 50, 25, 12.5, and 6.25 refer to 50, 25, 12.5, and 6.25 ng of recombinant OmpC/lane, respectively. (c) Flow cytometric analysis of recombinant L. casei. Bacterial cells labeled with anti-OmpC antibody and Alexa Fluor 488-conjugated IgG. Ten thousand events were analyzed and are shown in histogram form. The gray-shaded area represents LCN cells, and the nonshaded solid line represents LCO cells. (d) Evaluation of cell viability by flow cytometry. Bacterial cells (LCO or LCN) at exponential phase (8 h) and stationary phase (24 h) were stained with SYTO 9 and propidium iodide. The percentages of damaged and/or intact cells were calculated by using CellQuest software. The result shown is representative of two independent experiments.
Because pLP401 provides the signal peptide and anchor, LCO cells were also examined for whether the heterologous protein was located on the cell surface. As a result of flow cytometric analysis, the fluorescence intensity of labeled LCO cells shifted slightly (Fig. 1c). This result indicated that at least part of the OmpC protein was exposed on the bacterial cell surface.
In order to determine whether OmpC-expression affected the cell viability of recombinant strains, the bacterial cells at the exponential phase (8 h) and the stationary phase (24 h) were tested by the plate culture method and using a Live/Dead BacLight bacterial counting and viability kit. By plate culture, 1.47 CFU/particle were detected from the LCO culture, and 1.31 CFU/particle were observed from the LCN culture. Both CFU values were greater than 1, indicating that almost 100% of the cells were viable. The flow cytometric analysis supported this result because 98.7% of the LCO and 99.3% of the LCN cells clustered in the ICW/membrane region. A slightly broader cluster of intact LCO cells than LCN culture cells was observed at this growth phase. Meanwhile, LCO formed fewer colonies (0.83 CFU/particle) than LCN (1.35 CFU/particle) at the stationary phase. A relatively high ratio of membrane/wall-damaged cells was detected in the cytogram of LCO cells (8.3%), whereas almost all LCN cells clustered in the intact cell region.
OmpC-expressing recombinant L. casei induces less TNF-α release from RAW264.7 cells.
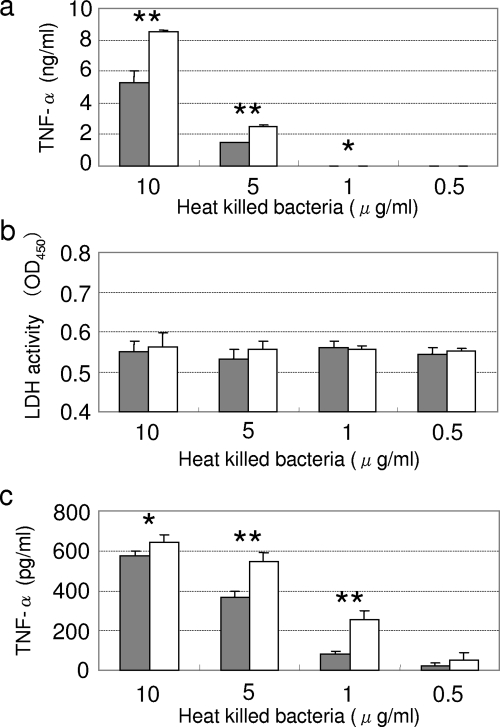
A murine macrophagelike cell line, RAW264.7, was stimulated with LCO cells, and TNF-α released into the culture supernatant was assayed. Cells incubated with heat-killed LCO cells released less TNF-α than did the nonexpressing L. casei strain in a dose-dependent manner (Fig. 2a). The lower cytokine induction was not caused by damage to the immune cells, because no difference was observed between LCO and LCN in the cytotoxicity assays (Fig. 2b). A regulatory cytokine, IL-10, was not detected in these cultures (data not shown). Similar results were obtained in the experiment using peritoneal macrophages instead of RAW264.7 cells (Fig. 2c).
FIG. 2.
TNF-α induction and LDH release caused by recombinant lactobacilli. (a) TNF-α released by RAW264.7 cells; (b) LDH release induced by recombinant L. casei; (c) TNF-α released by murine peritoneal macrophages. All three assays were performed using cell cultures incubated for 24 h. The concentrations of TNF-α in the culture supernatant or the OD450 are described in the left margin. The concentrations of heat-killed bacteria added to the cell cultures are shown in the bottom margin (in μg/ml). Solid bars represent LCO cells, and open bars represent LCN cells. The data are presented as the means plus the standard deviations (SD) (n = 3). The results shown are representative of three independent experiments. *, P < 0.05; **, P < 0.01.
Purified OmpC does not inhibit the proinflammatory response.
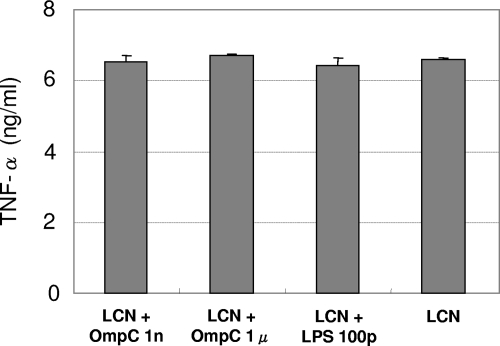
The possibility that OmpC inhibited the proinflammatory response of RAW264.7 cells was explored. Purified rOmpC was added to the cell culture with or without heat-killed LCN. Because rOmpC was prepared from E. coli, the level of LPS contamination was determined. The results of the LPS detection assay showed an LPS contamination level of ∼50 pg/ml in a 1-μg/ml solution of rOmpC, which could influence TNF-α production. In order to inhibit TNF-α induction by LPS, polymixin B nonapeptide was supplemented into the culture medium. The addition of 100 μg of polymixin B nonapeptide/ml eliminated <100 pg of LPS/ml completely but did not affect L. casei-induced TNF-α production (data not shown). As shown in Fig. 3, supplementing rOmpC did not inhibit LCN-induced TNF-α production, and no effect of LPS was observed.
FIG. 3.
TNF-α induction by LCN with or without rOmpC. A mixture of LCN (10 μg/ml) and rOmpC (or LPS) was added to a RAW264.7 cell culture. The concentrations of released TNF-α in the culture supernatant are described in the left margin. The data are presented as the means plus the SD (n = 3). The results shown are representative of three independent experiments. No significant difference was shown. 1n, 1 ng/ml; 1μ, μg/ml; 100p, 100 pg/ml.
Importance of phagocytosed bacteria to TNF-α production.
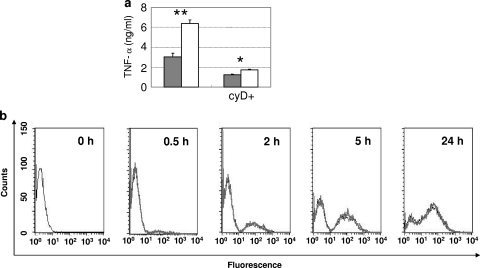
The importance of the internalization of bacteria in RAW264.7 cells was evaluated using cytochalasin D. After 24 h of incubation with the phagocytosis inhibitor, the accumulation of TNF-α in the culture supernatants was decreased remarkably (Fig. 4a).
FIG. 4.
Importance of phagocytosed bacteria in TNF-α induction and the frequency of phagocytosis. (a) TNF-α release elicited by recombinant lactobacilli with or without cytochalasin D (5 μg/ml) supplementation. Cytochalasin D was added to the RAW264.7 cell culture (described as cyD+) 30 min before the addition of 10 μg of LCO (solid bar) or 10 μg of LCN (open bar)/ml. The concentrations of released TNF-α (ng/ml) are indicated in the left margin. The data are presented as the mean plus the SD (n = 3). (b) FACS analysis of phagocytosis of lactobacilli. RAW264.7 cells were cultured with FITC-labeled bacteria (10 μg/ml) for 0, 0.5, 2, 5, or 24 h and then collected. The bold gray line and thin black line represent LCN and LCO, respectively. The results shown are representative of two independent experiments. *, P < 0.05; **, P < 0.01.
In order to investigate the efficiency of bacterial uptake by RAW264.7 cells, the cells exposed to heat-killed bacteria labeled with FITC were collected at different time points and applied to fluorescence-activated cell sorting (FACS) analysis. No remarkable difference between LCO and LCN was observed at any time point (Fig. 4b).
Differences in N-acetylmuramidase sensitivity.
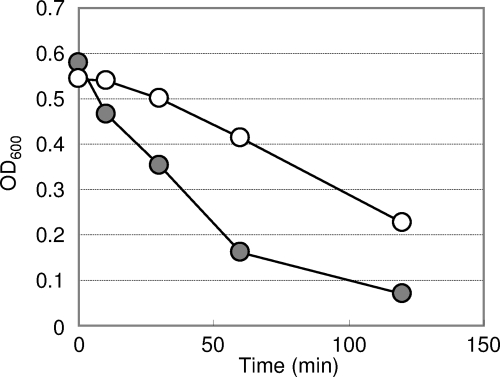
Internalized bacteria in macrophages are digested by phagolysosomal enzymes. If the bacteria resist such a digestive process, they may retain immunogenicity. To evaluate the sensitivity of recombinant lactobacilli to digestion, bacterial cells were treated with N-acetylmuramidase. As shown in Fig. 5, the lysis of LCO cells occurred more rapidly than that of LCN cells.
FIG. 5.
Evaluation of N-acetylmuramidase sensitivity. The reduction of the OD600 caused by cell lysis was measured at different time points. This result is representative of three independent experiments. Symbols: •, LCO cells; ○, LCN cells.
Differences in the sustainability of TNF-α induction.
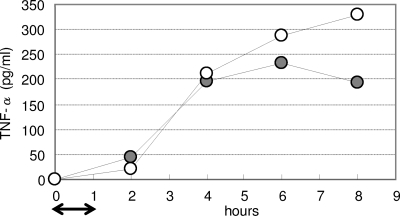
In order to determine the duration of cytokine production from RAW264.7 cells elicited by phagocytosed bacteria, culture supernatants from different time points were analyzed. After 1 h of uptake of bacteria, the same amount of TNF-α was released by macrophages stimulated with LCO and LCN until 4 h later; however, cells inoculated with LCO produced less TNF-α than with LCN after both 6- and 8-h incubations (Fig. 6).
FIG. 6.
Duration of TNF-α release induced by recombinant lactobacilli. LCO cells (•) or LCN cells (○) were added to a RAW264.7 cell culture, followed by incubation for 1 h (indicated by an arrow in the bottom margin) to allow phagocytosis. Excess bacteria that were not internalized were then removed by replacing the medium. Each culture supernatant was collected at 2, 4, 6, or 8 h. This result is representative of three independent experiments.
Cell wall involvement in differences in immunogenicity between LCO and LCN.
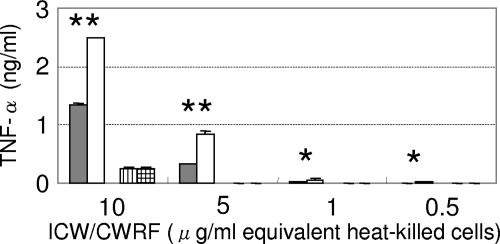
In order to identify the main component responsible for differences in immunogenicity between LCO and LCN, the ICW and CWRF of recombinant lactobacilli were prepared and added to the culture of RAW264.7 cells. As shown in Fig. 7, the cell wall of LCO elicited less inflammatory cytokine production than that of LCN, whereas both CWRFs stimulated macrophages weakly and equally.
FIG. 7.
TNF-α production induced by the ICW or CWRF of recombinant lactobacilli. Cell components were prepared from each concentration of heat-killed bacteria and added to RAW264.7 cell cultures. The concentrations of the added cell components described in the bottom margin are not the actual number but correspond to the concentration of heat-killed bacteria. The concentrations of released TNF-α are indicated in the left margin. The data are presented as the means + the SD (n = 3). The results are representative of two independent experiments. Bars: ▪, ICW from LCO cells; □, ICW from LCN cells; ▥, CWRF from LCO cells; , CWRF from LCN cells. *, P < 0.05; **, P < 0.01.
DISCUSSION
The heterologous expression of useful proteins in lactic acid bacteria has been trialed for medical applications. Many novel strains equipped supplemental function have been constructed by genetic modification. While the positive effects carried by the recombination were exhibited extensively in these studies, the negative effects caused by genetic modification have been scarcely remarked upon. The present study fortuitously found an unexpected negative effect of recombination caused by the expression of Salmonella OmpC in L. casei. As a purpose for which it was originally intended, a recombinant L. casei strain producing OmpC was established in order to develop a vaccine against Salmonella. Although the heterologous antigen was expressed clearly and exposed on the cell surface as a result of the use of an expression vector with a signal sequence and anchor, the efficiency of the surface localization seemed relatively low. Because Salmonella OmpC originally refers to a hydrophobic protein that forms channels in the outer membrane, it is understandable that the protein hardly passes through cytoplasmic membrane. Therefore, the result of FACS analysis for the detection of surface antigens led to speculation that only part of the produced antigens could pass through the cytoplasmic membrane, while the rest of the antigens were trapped around the membrane, or only the hydrophilic domain of the heterologous antigen was exposed on the cell surface, while the hydrophobic domain was left around the cell membrane. The expression of OmpC was also linked to the impaired cell viability of the recombinant lactobacilli. The data from flow cytometric analysis of recombinant bacteria stained with SYTO 9 and propidium iodide indicated that the cell membrane/wall of an OmpC-expressing strain was damaged. Because OmpC probably had high affinity to the cytoplasmic membrane, the protein might interrupt or affect cell membrane/wall formation. Among many genetically modified strains constructed in our laboratory, this is the only case in which such distinct damage to the cell envelope was observed using the pLP401 vector system (unpublished data).
The OmpC-expressing lactobacilli also exhibited side effects in their response to immune cells. It was found that the expression of SE OmpC reduced TNF-α production induced by L. casei from murine macrophages. Because none of the regulatory cytokines produced by macrophages, such as IL-10 (4, 8, 9), was detected during the incubation, this phenomenon may not occur by the downregulation of TNF-α expression. Subsequently, the study determined whether purified SE OmpC inhibited the release of TNF-α from RAW264.7 cells. Because 10 μg of OmpC-expressing L. casei, corresponding to approximately 5 × 106 CFU, was estimated to produce <1 ng of OmpC, 1 ng of OmpC/ml, along with a nonexpressing strain of L. casei, were added to the culture. A much higher concentration of OmpC (1 μg/ml) or higher contamination levels of LPS (100 pg/ml) were also supplemented; however, no significant differences were found. These results indicate that supplementation of purified recombinant SE OmpC into the cell culture did not prevent TNF-α induction by L. casei. This evidence does not support the phenomenon that the expression of SE OmpC by L. casei decreased the proinflammatory cytokine induction of RAW264.7 cells. The ICW prepared from OmpC-expressing L. casei still exhibited a different capacity from the nonexpressing strain to elicit TNF-α production by RAW264.7 cells. Because the ICW loses most protein during preparation, proteins may not affect this reaction. Taken together, OmpC, as a protein expressed by recombinant lactobacilli is not involved in the reduction of TNF-α release from RAW264.7 cells.
For further analysis, the present study evaluated the importance of phagocytosis in the immune responses of RAW264.7 cells stimulated with lactobacilli. Cytokine release by RAW264.7 cells was remarkably decreased by interference of phagocytosis by cytochalasin D, indicating that internalized bacteria mainly contribute to TNF-α induction. This evidence proposes a possibility in which the reduction in the number of internalized bacteria in macrophages could result in attenuation of the proinflammatory response. In order to determine the efficiency of phagocytosis, RAW264.7 cells internalizing FITC-labeled recombinant lactobacilli were analyzed by flow cytometry. However, the uptake frequency of recombinant bacteria was not different between OmpC-producing lactobacilli and the nonexpressing strain.
A phagosome including bacteria fuses to lysosomes and the bacteria are digested in the mature phagosome. In this process, N-acetylmuramidase, an enzyme that catalyzes the degradation of peptidoglycan, is one of the main agents for the digestion of bacteria (3). To evaluate the sensitivity to digestion, recombinant lactobacilli were treated with N-acetylmuramidase. As a result, the OmpC-expressing strain lysed more rapidly than the control strain. Consequently, the amount of TNF-α released from RAW264.7 cells was measured, and it was shown that the cells phagocytosed a certain amount of bacteria at several time points. During the time course, proinflammatory cytokines elicited by OmpC-producing L. casei terminated earlier than that by the control strain. These results suggest that the recombinant strain expressing the heterologous antigen may be digested more rapidly in macrophages and lose its immune-stimulating capability at an earlier time point. In this context, Shida et al. reported previously that Lactobacillus strains exhibiting relatively low sensitivity to N-acetylmuramidase show high potency to induce IL-12 (34). Hence, the robustness of bacterial cells is probably an important factor for their immunogenicity. Based on the above findings, the involvement of the cell wall was speculated in the attenuation of the proinflammatory response by OmpC-expressing L. casei. As expected, the ICW evoked TNF-α release strongly, while a cell wall-digested fraction induced low levels of cytokine production. Moreover, a significant difference between the recombinant strain producing OmpC and the control strain was observed with the cell wall. This result suggested that the expression of OmpC in L. casei may cause some sort of conversion in the structure of the cell wall. The specific structure affected by OmpC expression will be determined in a further study. In conclusion, OmpC expression in L. casei affected the N-acetylmuramidase sensitivity of the cell wall, which resulted in attenuation of the property to elicit a proinflammatory response in RAW264.7 cells.
Recently, genetically modified lactobacilli have been developed for vaccines, anti-allergic agents, and other applications. Among these studies, reduction or negative effects brought about by heterologous gene expression have not yet been reported. The attenuation of immunogenicity due to OmpC expression is probably a unique and exceptional phenomenon; however, the present study provides important information about the pleiotropic effects of genetic modifications.
Acknowledgments
This study was supported by a grant from the Ministry of Health, Labor, and Welfare (Research on Food Safety) and partly by a grant from the Food Safety Commission of Japan.
Footnotes
Published ahead of print on 6 March 2009.
REFERENCES
- 1.Acedo-Félix, E., and G. Pérez-Martínez. 2003. Significant differences between Lactobacillus casei subsp. casei ATCC 393T and a commonly used plasmid-cured derivative revealed by a polyphasic study. Int. J. Syst. Evol. Microbiol. 53:67-75. [DOI] [PubMed] [Google Scholar]
- 2.Aires, K. A., A. M. Cianciarullo, S. M. Carneiro, L. L. Villa, E. Boccardo, G. Pérez-Martinez, I. Perez-Arellano, M. L. Oliveira, and P. L. Ho. 2006. Production of human papillomavirus type 16 L1 virus-like particles by recombinant Lactobacillus casei cells. Appl. Environ. Microbiol. 72:745-752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Akporiaye, E. T., J. D. Rowatt, A. A. Aragon, and O. G. Baca. 1983. Lysosomal response of a murine macrophage-like cell line persistently infected with Coxiella burnetii. Infect. Immun. 40:1155-1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bogdan, C., Y. Vodovotz, and C. Nathan. 1991. Macrophage deactivation by interleukin 10. J. Exp. Med. 174:1549-1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Corthésy, B., S. Boris, P. Isler, C. Grangette, and A. Mercenier. 2005. Oral immunization of mice with lactic acid bacteria producing Helicobacter pylori urease B subunit partially protects against challenge with Helicobacter felis. J. Infect. Dis. 192:1441-1449. [DOI] [PubMed] [Google Scholar]
- 6.Daniel, C., A. Repa, C. Wild, A. Pollak, B. Pot, H. Breiteneder, U. Wiedermann, and A. Mercenier. 2006. Modulation of allergic immune responses by mucosal application of recombinant lactic acid bacteria producing the major birch pollen allergen Bet v 1. Allergy 61:812-819. [DOI] [PubMed] [Google Scholar]
- 7.Danner, R. L., K. A. Joiner, M. Rubin, W. H. Patterson, N. Johnson, K. M. Ayers, and J. E. Parrillo. 1989. Purification, toxicity, and antiendotoxin activity of polymyxin B nonapeptide. Antimicrob. Agents Chemother. 33:1428-1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Waal Malefyt, R., J. Abrams, B. Bennett, C. G. Figdor, and J. E. de Vries. 1991. Interleukin 10 (IL-10) inhibits cytokine synthesis by human monocytes: an autoregulatory role of IL-10 produced by monocytes. J. Exp. Med. 174:1209-1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fiorentino, D. F., A. Zlotnik, T. R. Mosmann, M. Howard, and A. O'Garra. 1991. IL-10 inhibits cytokine production by activated macrophages. J. Immunol. 147:3815-3822. [PubMed] [Google Scholar]
- 10.Franchi, L., J. H. Park, M. H. Shaw, N. Marina-Garcia, G. Chen, Y. G. Kim, and G. Núñez. 2008. Intracellular NOD-like receptors in innate immunity, infection, and disease. Cell. Microbiol. 10:1-8. [DOI] [PubMed] [Google Scholar]
- 11.Fujiwara, D., S. Inoue, H. Wakabayashi, and T. Fujii. 2004. The anti-allergic effects of lactic acid bacteria are strain dependent and mediated by effects on both Th1/Th2 cytokine expression and balance. Int. Arch. Allergy Immunol. 135:205-215. [DOI] [PubMed] [Google Scholar]
- 12.Grangette, C., H. Müller-Alouf, D. Goudercourt, M. C. Geoffroy, M. Turneer, and A. Mercenier. 2001. Mucosal immune responses and protection against tetanus toxin after intranasal immunization with recombinant Lactobacillus plantarum. Infect. Immun. 69:1547-1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grangette, C., S. Nutten, E. Palumbo, S. Morath, C. Hermann, J. Dewulf, B. Pot, T. Hartung, P. Hols, and A. Mercenier. 2005. Enhanced antiinflammatory capacity of a Lactobacillus plantarum mutant synthesizing modified teichoic acids. Proc. Natl. Acad. Sci. USA 102:10321-10326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hou, X. L., L. Y. Yu, J. Liu, and G. H. Wang. 2007. Surface-displayed porcine epidemic diarrhea viral (PEDV) antigens on lactic acid bacteria. Vaccine 26:24-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Isibasi, A., V. Ortiz, M. Vargas, J. Paniagua, C. González, J. Moreno, and J. Kumate. 1988. Protection against Salmonella typhi infection in mice after immunization with outer membrane proteins isolated from Salmonella typhi 9,12,d,Vi. Infect. Immun. 56:2953-2959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Isibasi, A., V. Ortiz-Navarrete, J. Paniagua, R. Pelayo, C. R. González, J. A. García, and J. Kumate. 1992. Active protection of mice against Salmonella typhi by immunization with strain-specific porins. Vaccine 10:811-813. [DOI] [PubMed] [Google Scholar]
- 17.Kajikawa, A., E. Satoh, R. J. Leer, S. Yamamoto, and S. Igimi. 2007. Intragastric immunization with recombinant Lactobacillus casei expressing flagellar antigen confers antibody-independent protective immunity against Salmonella enterica serovar Enteritidis. Vaccine 25:3599-3605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim, Y. G., T. Ohta, T. Takahashi, A. Kushiro, K. Nomoto, T. Yokokura, N. Okada, and H. Danbara. 2006. Probiotic Lactobacillus casei activates innate immunity via NF-κB and p38 MAP kinase signaling pathways. Microbes Infect. 8:994-1005. [DOI] [PubMed] [Google Scholar]
- 19.Kruisselbrink, A., M. J. Heijne den Bak-Glashouwer, C. E. Havenith, J. E. Thole, and R. Janssen. 2001. Recombinant Lactobacillus plantarum inhibits house dust mite-specific T-cell responses. Clin. Exp. Immunol. 126:2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kuusi, N., M. Nurminen, H. Saxen, M. Valtonen, and P. H. Mäkelä. 1979. Immunization with major outer membrane proteins in experimental salmonellosis of mice. Infect. Immun. 25:857-862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee, J. S., H. Poo, D. P. Han, S. P. Hong, K. Kim, M. W. Cho, E. Kim, M. H. Sung, and C. J. Kim. 2006. Mucosal immunization with surface-displayed severe acute respiratory syndrome coronavirus spike protein on Lactobacillus casei induces neutralizing antibodies in mice. J. Virol. 80:4079-4087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maassen, C. B., J. D. Laman, M. J. den Bak-Glashouwer, F. J. Tielen, J. C. van Holten-Neelen, L. Hoogteijling, C. Antonissen, R. J. Leer, P. H. Pouwels, W. J. Boersma, and D. M. Shaw. 1999. Instruments for oral disease-intervention strategies: recombinant Lactobacillus casei expressing tetanus toxin fragment C for vaccination or myelin proteins for oral tolerance induction in multiple sclerosis. Vaccine 17:2117-2128. [DOI] [PubMed] [Google Scholar]
- 23.Maassen, C. B., J. D. Laman, C. van Holten-Neelen, L. Hoogteijling, L. Groenewegen, L. Visser, M. M. Schellekens, W. J. Boersma, and E. Claassen. 2003. Reduced experimental autoimmune encephalomyelitis after intranasal and oral administration of recombinant lactobacilli expressing myelin antigens. Vaccine 21:4685-4693. [DOI] [PubMed] [Google Scholar]
- 24.Matsuguchi, T., A. Takagi, T. Matsuzaki, M. Nagaoka, K. Ishikawa, T. Yokokura, and Y. Yoshikai. 2003. Lipoteichoic acids from Lactobacillus strains elicit strong tumor necrosis factor alpha-inducing activities in macrophages through Toll-like receptor 2. Clin. Diagn. Lab. Immunol. 10:259-266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mercenier, A., S. Pavan, and B. Pot. 2003. Probiotics as biotherapeutic agents: present knowledge and future prospects. Curr. Pharm. Des. 9:175-191. [DOI] [PubMed] [Google Scholar]
- 26.Nikaido, H., and M. Vaara. 1985. Molecular basis of bacterial outer membrane permeability. Microbiol. Rev. 49:1-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Oliveira, M. L., A. P. Arêas, I. B. Campos, V. Monedero, G. Perez-Martínez, E. N. Miyaji, L. C. Leite, K. A. Aires, and P. Lee Ho. 2006. Induction of systemic and mucosal immune response and decrease in Streptococcus pneumoniae colonization by nasal inoculation of mice with recombinant lactic acid bacteria expressing pneumococcal surface antigen A. Microbes Infect. 8:1016-1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Perdigón, G., C. Maldonado Galdeano, J. C. Valdez, and M. Medici. 2002. Interaction of lactic acid bacteria with the gut immune system. Eur. J. Clin. Nutr. 56(Suppl. 4):S21-S26. [DOI] [PubMed] [Google Scholar]
- 29.Perea Vélez, M., T. L. Verhoeven, C. Draing, S. von Aulock, M. Pfitzenmaier, A. Geyer, I. Lambrichts, C. Grangette, B. Pot, J. Vanderleyden, and S. C. de Keersmaecker. 2007. Functional analysis of d-alanylation of lipoteichoic acid in the probiotic strain Lactobacillus rhamnosus GG. Appl. Environ. Microbiol. 73:3595-3604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Poo, H., H. M. Pyo, T. Y. Lee, S. W. Yoon, J. S. Lee, C. J. Kim, M. H. Sung, and S. H. Lee. 2006. Oral administration of human papillomavirus type 16 E7 displayed on Lactobacillus casei induces E7-specific antitumor effects in C57/BL6 mice. Int. J. Cancer 119:1702-1709. [DOI] [PubMed] [Google Scholar]
- 31.Pouwels, P. H., A. Vriesema, B. Martinez, F. J. Tielen, J. F. Seegers, R. J. Leer, J. Jore, and E. Smit. 2001. Lactobacilli as vehicles for targeting antigens to mucosal tissues by surface exposition of foreign antigens. Methods Enzymol. 336:369-389. [DOI] [PubMed] [Google Scholar]
- 32.Scheppler, L., M. Vogel, P. Marti, L. Müller, S. M. Miescher, and B. M. Stadler. 2005. Intranasal immunization using recombinant Lactobacillus johnsonii as a new strategy to prevent allergic disease. Vaccine 23:1126-1134. [DOI] [PubMed] [Google Scholar]
- 33.Shaw, D. M., B. Gaerthé, R. J. Leer, J. G. van der Stap, C. Smittenaar, M. Heijne den Bak-Glashouwer, J. E. Thole, F. J. Tielen, P. H. Pouwels, and C. E. Havenith. 2000. Engineering the microflora to vaccinate the mucosa: serum immunoglobulin G responses and activated draining cervical lymph nodes following mucosal application of tetanus toxin fragment C-expressing lactobacilli. Immunology 100:510-518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shida, K., J. Kiyoshima-Shibata, M. Nagaoka, K. Watanabe, and M. Nanno. 2006. Induction of interleukin-12 by lactobacillus strains having a rigid cell wall resistant to intracellular digestion. J. Dairy Sci. 89:3306-3317. [DOI] [PubMed] [Google Scholar]
- 35.Shimosato, T., H. Kitazawa, S. Katoh, M. Tohno, I. D. Iliev, C. Nagasawa, T. Kimura, Y. Kawai, and T. Saito. 2005. Augmentation of T(H)-1 type response by immunoactive AT oligonucleotide from lactic acid bacteria via Toll-like receptor 9 signaling. Biochem. Biophys. Res. Commun. 326:782-787. [DOI] [PubMed] [Google Scholar]