FIG. 4.
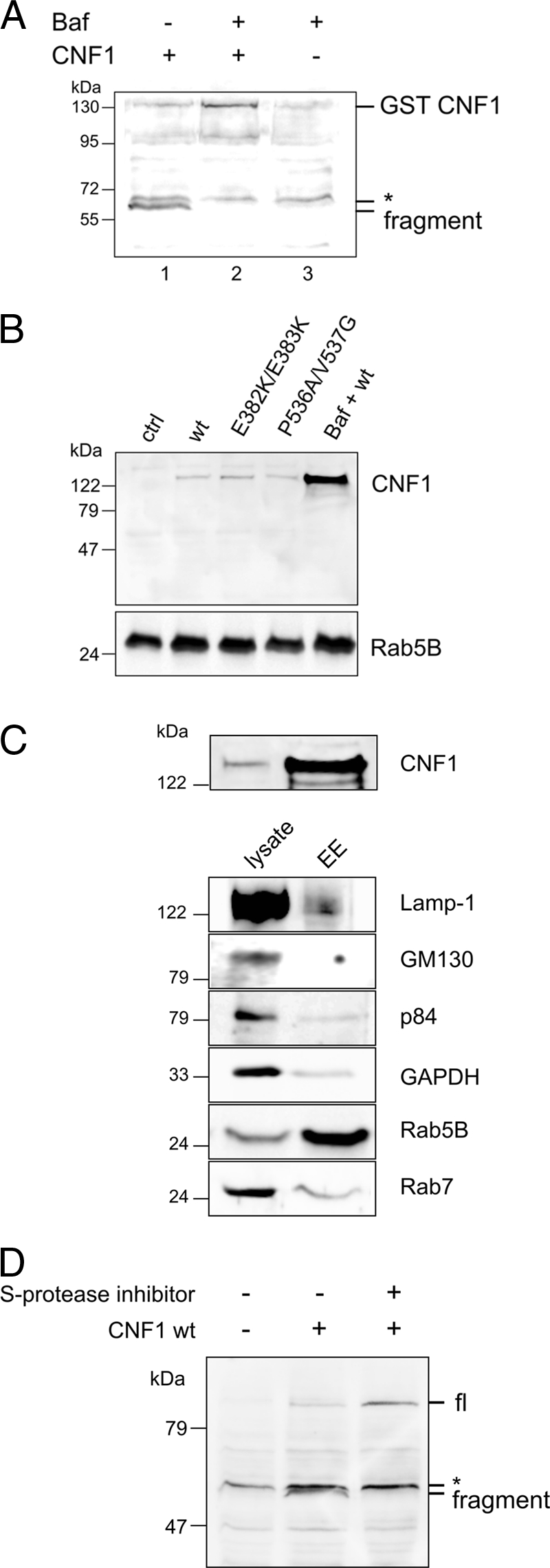
CNF1 is not cleaved in the cytosol (A). CNF1 (1 μg/ml) was bound to HeLa cells for 2 h at 4°C in the absence (lane 1) or in the presence (lane 2) of bafilomycin A1, which blocks endosome-derived uptake of the toxin. After this, cells were washed with Hanks balanced salt solution (pH 4.8) and incubated at 37°C in the buffer for 10 min (lane 2) or left untreated (lane 1). Then cells were incubated in DMEM for 4 h at 37°C in the absence (lane 1) or in the presence (lane 2) of bafilomycin A1 (100 nM). Cell lysate was separated into cytosolic and membrane fractions, and the cytosolic fraction was analyzed for full-length or cleaved CNF1 by Western blotting with an antibody against CNF1. Lane 3 contained a control with bafilomycin A1 but without CNF1. Note that a nonspecific band above the CNF1 fragment band was detected by the antibody (asterisk). In bafilomycin A1-treated cells CNF1 remained uncleaved in endosomes (B). HeLa cells were treated with CNF1 and CNF1 mutants (400 ng/ml) in the absence or presence of bafilomycin A1 (100 nM) overnight. Endosomes of CNF1-treated cells (lanes 2 to 5) and control cells (lane 1) were purified, and the endosomal fraction was separated by SDS-PAGE. CNF1 was detected with an antibody against the C terminus (B). For control of endosome enrichment, the presence of the early endosome marker Rab5B in the corresponding endosomes was analyzed. In endosomes of cells pretreated with bafilomycin A1, large amounts of CNF1 were detected, whereas in the absence of bafilomycin only traces remained in the endosomes of cells intoxicated with wild-type CNF1, CNF1(E382K/E383K) (pore-formation-deficient mutant), and CNF1(P536A/V537G) (cleavage site mutant). (C) Purity of early endosomes from HeLa cells. Fifteen-microgram portions of the lysates of CNF1-treated cells and the corresponding purified endosomes were separated by SDS-PAGE and blotted to determine the presence of CNF1 and specific marker proteins, including Lamp-1 (lysosome), GM130 (Golgi apparatus), p84 (nucleus), glyceraldehyde-3-phosphate dehydrogenase (cytosol), Rab5B (early endosome), and Rab7 (late endosome). (D) In the presence of serine protease inhibitors CNF1 remained uncleaved (full length). HeLa cells were treated with wild-type CNF1 (800 ng/ml) in the absence or presence of a serine protease inhibitor cocktail as indicated for 1 h at 37°C and lysed. Lysates were analyzed to determine the presence of cleaved and uncleaved (full-length) CNF1 in a Western blot against CNF1. Note that a nonspecific band above the CNF1 fragment band was detected by the antibody (asterisk). The experiments were repeated three times with similar results. Baf, bafilomycin A1; GST, glutathione S-transferase; wt, wild-type CNF1; EE, enriched endosomes; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; S-protease inhibitor, serine protease inhibitor cocktail; fl, full length.
