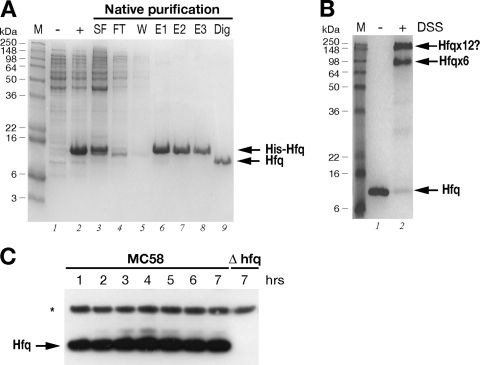
FIG. 2.
(A) Expression and purification of the recombinant Hfq protein. SDS-PAGE was performed with protein extracts from uninduced (lane −) and IPTG-induced (lane +) cultures of E. coli containing the pET15bHfq expression plasmid, the cleared soluble fraction before (lane SF) and after (lane FT) binding to the Ni-NTA resin, the wash fraction (lane W), elution fractions (lanes E1 to E3), and untagged Hfq protein after thrombin digestion (lane Dig). (B) In vitro cross-linking of Hfq reveals hexameric forms in solution. For in vitro cross-linking, the untagged protein was not treated (lane −) or treated (lane +) with the cross-linking reagent DSS. The relative positions of the molecular weight markers (lane M) are indicated on the left. The positions of the oligomeric forms of the Hfq protein are indicated on the right. (C) Western blot showing the expression of the Hfq protein in wild-type strain MC58 or the Δhfq mutant over time. Total protein samples were taken at the time points shown in Fig. 3A. Five micrograms of total protein was loaded for each time point. Anti-Hfq antiserum recognizes a band at approximately 11 kDa in the wild-type strain but not in the Hfq null mutant. Antiserum against NMB2091 was also used to stain the blot as a loading control for total protein, as indicated by the asterisk.