Abstract
DNA microarray analysis revealed that transcription of the Bacillus subtilis yetM gene encoding a putative flavin adenine dinucleotide-dependent monooxygenase was triggered by certain flavonoids during culture and was derepressed by disruption of the yetL gene in the opposite orientation situated immediately upstream of yetM, which encodes a putative MarR family transcriptional regulator. In vitro analyses, including DNase I footprinting and gel retardation analysis, indicated that YetL binds specifically to corresponding single sites in the divergent yetL and yetM promoter regions, with higher affinity to the yetM region; the former region overlaps the Shine-Dalgarno sequence of yetL, and the latter region contains a perfect 18-bp palindromic sequence (TAGTTAGGCGCCTAACTA). In vitro gel retardation and in vivo lacZ fusion analyses indicated that some flavonoids (kaempferol, apigenin, and luteolin) effectively inhibit YetL binding to the yetM cis sequence, but quercetin, galangin, and chrysin do not inhibit this binding, implying that the 4-hydroxyl group on the B-ring of the flavone structure is indispensable for this inhibition and that the coexistence of the 3-hydroxyl groups on the B- and C-rings does not allow antagonism of YetL.
The rhizosphere is the surface region of soil that is directly influenced by root secretions and associated soil microorganisms. A large population of bacteria is present in the rhizosphere, where the bacteria are able to feed on nutrients released from plant cells, such as sugars, amino acids, and lipids, and they survive coordinately or hostilely with each other according to the environment in which they live (3).
Similar to nutrient material, flavonoids are exuded by plant cells, and therefore they are abundant in the soil, especially in the rhizosphere. Certain flavonoids possess antibacterial activity; quercetin inhibits bacterial DNA gyrase, which induces DNA cleavage (20, 33). To avoid such harmful effects, some bacteria have a system for degradation of flavonoids that detoxifies them (22). A gram-positive soil bacterium, Bacillus subtilis, possesses a quercetin 2,3-dioxygenase that converts quercetin to 2-protocatechuoyl-phloroglucinol carboxylic acid and carbon monoxide (4). So far, quercetin 2,3-dioxygenase has been isolated from several bacteria and fungi (12, 17); hence, this enzyme appears to be widely distributed and to play a major role in flavonoid degradation in soil microorganisms.
In B. subtilis, the yxaG gene encoding quercetin 2,3-dioxygenase is a member of an operon containing the yxaH gene encoding a membrane protein with an unknown function (4, 38). Our previous study demonstrated that the yxaGH operon is regulated by two paralogous transcriptional regulators, LmrA and YxaF, in response to certain flavonoids (9). LmrA and YxaF, both of which belong to the TetR family, similarly recognize and bind to the two cis sequences (LmrA/YxaF boxes) located tandemly in the yxaGH promoter region, and the binding of these two regulators is inhibited efficiently and distinctly by flavonoids, such as quercetin and fisetin; in this way transcription is induced. The lmrA gene is the first gene in the lmrAB operon, and the product of the second gene, lmrB, is a member of the major facilitator superfamily involved in resistance to several drugs, such as lincomycin and puromycin (19). The yxaF gene is located immediately upstream of the yxaGH operon and is oriented in the same direction as yxaGH (38). LmrA and YxaF also regulate the lmrAB operon and the yxaF gene, binding to and becoming detached from the corresponding single LmrA/YxaF boxes in their promoter regions, as is the case for yxaGH (9).
It is intriguing that B. subtilis utilizes flavonoids as signaling molecules to induce resistance to structurally unrelated antibiotics, such as lincomycin and puromycin, through the LmrA/YxaF regulation system. We assume that this might be one of the strategies that B. subtilis uses in its struggle against other microorganisms in the mixed microbiological flora in the rhizosphere, the environmental conditions of which B. subtilis perceives through the abundant flavonoids (26). A similar situation was observed for the habitat of Staphylococcus aureus, in which gene expression for the QacA major facilitator superfamily pump controlled by QacR, a member of the TetR family, is induced in response to the plant alkaloid berberine (5).
LmrA and YxaF were the first characterized flavonoid- responsive regulators in the genus Bacillus. On the other hand, NodD regulators, which belong to the LysR family and control transcription of the nod operons involved in nodulation of Rhizobiales in response to flavonoid signals released by the leguminous hosts, have been characterized in detail (13). Also, in Pseudomonas putida DOT-T1E, the resistance-nodulation-cell division family transporter TtgABC and the cognate TetR family repressor TtgR constitute a multidrug recognition system, and several flavonoids are substrates of TtgABC and trigger pump expression through binding to the TtgR-operator complex to dissociate it (30). Since it is not rare for flavonoids to function as signaling molecules for communication among soil bacteria and plants, it was expected that, in addition to the LmrA/YxaF regulon, B. subtilis possesses genes involved in flavonoid degradation or another physiological function for intercellular communication via flavonoids, which are under the control of unknown transcriptional regulators in response to flavonoids.
In this study, in order to elucidate the comprehensive regulatory system for the expression of the genes responsive to flavonoids in B. subtilis, we tried to identify additional genes that are significantly induced by flavonoid addition by means of DNA microarray analysis. Among the new candidate flavonoid-inducible genes found, we focused on the yetM gene encoding a putative flavin adenine dinucleotide (FAD)-dependent monooxygenase and on its transcriptional regulatory mechanism. DNA microarray analysis involving the wild-type strain and a yetL disruptant, performed in the framework of the Japan Functional Analysis Network for B. subtilis (JAFAN) (http://bacillus.genome.jp/), suggested that the product of the yetL gene, which encodes a putative transcriptional regulator of the MarR family and is located immediately upstream of the yetM gene in the opposite direction, negatively regulates yetM transcription, which is induced by certain flavonoids. DNA binding experiments involving recombinant YetL showed that YetL binds to the corresponding single sites in the yetL and yetM promoter regions, with particularly higher affinity for the latter region. The DNA binding of YetL was inhibited effectively by flavonoids such as kaempferol, apigenin, and luteolin, and its weaker interaction with flavonoids such as quercetin and fisetin appears to be different from the interaction of LmrA/YxaF. To date, the flavonoid-responsive transcriptional regulators of several microorganisms have been reported. However, to our knowledge, this is the first demonstration that a MarR family member specifically responds to flavonoids, which provides a clue for elucidation of the entire regulatory mechanism for flavonoid-induced gene expression.
MATERIALS AND METHODS
B. subtilis strains and their construction and cultivation.
The B. subtilis strains used in this study are listed in Table 1. B. subtilis strain 168 was used as the standard strain (wild type). Strain YETLd was constructed by integration of plasmid pMUTIN2 (34) into the yetL gene of strain 168 (14; http://bacillus.genome.jp/).
TABLE 1.
B. subtilis strains used in this study
| Strain | Genotype | Reference |
|---|---|---|
| 168 | trpC2 (standard strain, wild type) | |
| YETLd | yetL::pMUTIN2 trpC2 | 14 |
| FU1033 | ΔyetL::cat trpC2 | This study |
| FU1034 | ΔyetL::tet trpC2 | This study |
| FU1035 | amyE::[cat PyetL(−188 to 28)-lacZ] trpC2 | This study |
| FU1036 | amyE::[cat PyetL(−334 to 228)-lacZ] trpC2 | This study |
| FU1037 | amyE::[cat PyetM(−313 to 249)-lacZ] trpC2 | This study |
| FU1038 | ΔyetL::tet amyE::[cat PyetL(−188 to 28)-lacZ] trpC2 | This study |
| FU1039 | ΔyetL::tet amyE::[cat PyetL(−334 to 228)-lacZ] trpC2 | This study |
| FU1040 | ΔyetL::tet amyE::[cat PyetM(−313 to 249)-lacZ] trpC2 | This study |
Strain FU1033 carrying a yetL deletion was constructed as follows. Long-flanking homology PCR (35) was performed to create a DNA fragment in which the chloramphenicol acetyltransferase gene (cat) (11) was in the same orientation as the original yetL gene and sandwiched by the upstream and downstream regions of the yetL gene. The regions upstream and downstream of yetL were both amplified by PCR with the genomic DNA of strain 168 as the template and two primer pairs (yetLupF1/yetLupR_catup and yetLdownF_catdown/yetLdownR1 [Table 2], respectively). The cat cassette was amplified by PCR with primer pair catF/catR (Table 2) and plasmid pCBB31 bearing the cat gene (24) as the template. The three PCR products described above were added to a reaction mixture containing an ExTaq DNA polymerase (Takara-bio, Japan) and deoxynucleoside triphosphates without any primer oligonucleotide, and then denaturation, annealing, and extension reactions were carried out to combine the three fragments. Nested PCR with the resultant fragment as the template and primer pair yetLupF2/yetLdownR2 (Table 2) was performed to amplify the combined DNA fragment, which was then used to transform strain 168 to chloramphenicol resistance (5 μg/ml), which yielded strain FU1033 (ΔyetL::cat) (Table 1). Correct replacement of the yetL gene with cat was confirmed by PCR and DNA sequencing. Strain FU1033 was transformed with plasmid pCm::Tc (28) to change the chloramphenicol resistance to tetracycline resistance (10 μg/ml), which yielded strain FU1034 (ΔyetL::tet) (Table 1).
TABLE 2.
Oligonucleotide primers used in this study
| Primer | Sequence (5′-3′)b |
|---|---|
| yetLupF1 | CACGAACAACGGAGTGAATGCC |
| yetLupR_catup | CTAATGGGTGCTTTAGTTGAAGACGCAATTAGCTCCCGTACATTC |
| yetLdownF_catdown | GAGATAATGCCGACTGTACTGTCCGATCCGGACAGGCTATAGC |
| yetLdownR1 | CCATGTTGATCGGTATTTTATCC |
| catFa | TCTTCAACTAAAGCACCCATTAG |
| catRa | AGTACAGTCGGCATTATCTC |
| yetLupF2 | GTCAAAGCCCGCCAGAATGTCAG |
| yetLdownR2 | GACGCTGATCGGCATTTTGCTGGC |
| PyetL_PEF | GCTCTAGATCAGCTCTATATG |
| PyetL_PER | CGGGATCCCGCAATTAGCTCCC |
| PyetL_200F | GCTCTAGAAAGCCGTGCTTACAGCAATC |
| PyetL_200R | CGGGATCCGCATCAAAGAGCAGCATCAG |
| PyetM_200F | GCTCTAGAGCATCAAAGAGCAGCATCAG |
| PyetM_200R | CGGGATCCAAGCCGTGCTTACAGCAATC |
| PEpFa | CCAGTTAAAGGATTTGAGCGTAGCGAA |
| PEpRa | TCCACAGTAGTTCACCACCTTTTCCCTATA |
| PyetLF | CCCAAGAAAAGCTGTCAATC |
| PyetLR | CCTCGGCCAGCAAAGTAATG |
| PyetMF | GACACGATTATAAAGGAGGG |
| PyetMR | CATAAGGTGACGGAAAACCC |
| PyetL_delEF | AATCGTGTCACAACGAATGTTGCGATGGAATTAAAACATT |
| PyetL_delER | AATGTTTTAATTCCATCGCAACATTCGTTGTGACACGATT |
| yetLORF_NF | ATTCATATGGAATTAAAACATTTACCG |
| yetLORF_BR | TCCGGATCCGACGGGTTTTTTAGTCTTTAAAC |
To construct strain FU1035 carrying the yetL promoter region (bases −118 to 28) fused to the lacZ reporter gene and strains FU1036 and FU1037, both of which carried a fragment covering 200 bp of the open reading frame (ORF) of yetL, the entire intergenic region between yetL and yetM, and 200 bp of the yetM ORF fused to the lacZ gene in the opposite orientation (bases −334 to 228 for the yetL promoter and bases −313 to 249 for the yetM promoter; base 1 was the transcription start site for each of the sequences identified in this work), the corresponding regions were amplified by PCR with genomic DNA of strain 168 as the template and primer pairs PyetL_PEF/PyetL_PER, PyetL_200F/PyetL_200R, and PyetM_200F/PyetM_200R, respectively (Table 2). Each of the PCR products, trimmed by XbaI and BamHI digestion, was cloned into the pCRE-test2 vector (18), which had been treated with the same restriction enzymes. Correct construction was confirmed by DNA sequencing. The resultant plasmids were linearized by PstI digestion and then integrated into the amyE locus of strain 168 through double-crossover transformation to obtain chloramphenicol resistance, which resulted in strains FU1035, FU1036, and FU1037 (Table 1), respectively.
Strains FU1035, FU1036, and FU1037 were transformed with the genomic DNA of strain FU1034 (ΔyetL::tet) to obtain tetracycline resistance, which resulted in strains FU1038, FU1039, and FU1040 (Table 1), respectively.
B. subtilis cells were pregrown on tryptose blood agar base (Difco) plates supplemented with 0.18% glucose containing chloramphenicol (5 μg/ml), erythromycin (0.3 μg/ml), and/or tetracycline (10 μg/ml) according to the drug resistance of the cells at 30°C overnight. The cells were inoculated into Luria-Bertani (LB) medium (23) or minimal medium containing 0.4% glucose, 0.2% glutamine, and 50 μg/ml tryptophan (MM medium) (38) supplemented with a mixture of 16 amino acids (glutamine, histidine, tyrosine, and aspargine were omitted) (2) to obtain an optical density at 600 nm (OD600) of 0.05 and then incubated at 37°C with shaking.
DNA microarray analysis.
DNA microarray analysis was performed as described previously (39). Strain 168 cells were cultivated at 37°C in 200 ml of MM medium supplemented with 16 amino acids as described above until the OD600 reached 0.2, and either quercetin or fisetin dissolved in dimethyl sulfoxide (DMSO) was added to the medium at a final concentration of 200 μg/ml. The same volume of DMSO that was added to the flavonoid solution was added to a control culture. After further cultivation until the OD600 reached 0.8, the cells were harvested by centrifugation, and then total RNA was extracted and purified for synthesis of cDNA labeled with a fluorescent dye (Cy5 for the flavonoid treatment sample and Cy3 for the control).
Primer extension analysis.
Two sets of strains, strains FU1035 and FU1038 and strains 168 and YETLd, were used for primer extension analysis to determine the transcription start sites of the yetL and yetM genes, respectively. Cells of each strain were grown in LB medium until the OD600 reached 1.0 and harvested, and then total RNA was extracted and purified as described previously (39). For the primer extension reaction for the yetL and yetM transcripts, total RNA (45 μg) was annealed to 1 pmol each of primers PEpR and PyetMR (Table 2), respectively, which had been 5′ end labeled with a MEGALABEL kit (Takara-bio) and [γ-32P]ATP (MP Biomedicals), and then the primer extension reaction was conducted with ThermoScript reverse transcriptase (Invitrogen) as described previously (24). Templates for the dideoxy sequencing reactions for ladder preparation, starting with the same 5′-end-labeled primers that were used for yetL and yetM reverse transcription, were generated by PCR with genomic DNA of strains FU1035 and 168 as the templates and primer pairs PEpF/PEpR and PyetMF/PyetMR, respectively (Table 2). Autoradiograms were obtained and quantified using a Typhoon 9400 variable image analyzer (GE Healthcare).
Production and purification of the YetL protein.
The yetL ORF was amplified by PCR with genomic DNA of B. subtilis strain 168 as the template and primer pair yetLORF_NF/yetLORF_BR (Table 2), digested with NdeI and BamHI, and then cloned into the pET-22b(+) vector (Novagen) which had been treated with the same restriction enzymes, which yielded an expression plasmid, pET-YetL. Correct cloning of the yetL gene was confirmed by DNA sequencing.
Escherichia coli strain BL21(DE3) transformed with pET-YetL was grown in LB medium supplemented with ampicillin (50 μg/ml) at 37°C to an OD600 of 0.4. After isopropyl-β-d-thiogalactopyranoside (IPTG) was added to a final concentration of 1 mM, the cells were cultivated for another 3 h. The cells harvested from 4 liters of the culture were disrupted by sonication in 20 mM Tris-Cl buffer (pH 8.0) containing 10% (vol/vol) glycerol, 0.1 mM phenylmethylsulfonyl fluoride, and 1 mM dithiothreitol. After centrifugation (17,000 × g, 4°C, 20 min) and filtration (0.45 μm), the supernatant was recovered and subjected to (NH4)2SO4 precipitation. The supernatant fraction at 70% saturation was dialyzed against the same buffer that was used for sonication and then applied to a DEAE-Toyo-Pearl 650 M column (Tosoh, Japan) equilibrated with 20 mM Tris-Cl buffer (pH 8.0) containing 10% (vol/vol) glycerol. The column was washed with the same buffer that was in the column and was eluted with a linear 0 to 1 M NaCl gradient in the same buffer. The YetL fraction was collected and concentrated by ultrafiltration. The homogeneity of the YetL protein was confirmed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (PAGE) and staining with Coomassie brilliant blue. The purified YetL protein was subjected to gel filtration (TSK gel G3000 SWXL column; 7.8 mm by 30 cm; Tosoh) with 0.1 M potassium phosphate buffer (pH 7.0) containing 0.1 M Na2SO4 and 0.05% (wt/vol) NaN3 at a flow rate of 0.2 ml/min to determine the molecular mass of the native form of YetL.
DNase I footprinting analysis.
DNase I footprinting analysis was performed as described previously (9). The PyetL and PyetM probes used for footprinting were prepared by PCR with genomic DNA of strain 168 and primer pairs PyetLF/PyetLR and PyetMF/PyetMR (Table 2), respectively. Prior to PCR amplification, only the 5′ terminus of one of the primers was labeled with [γ-32P]ATP using a MEGALABEL kit. The DNA probe (0.04 pmol) labeled at the 5′ end was mixed with the YetL protein prepared as described above to obtain a DNA-protein complex, which was then partially digested with DNase I (Takara-bio) in 50 μl of the reaction mixture, and this was followed by urea-PAGE with sequencing ladders prepared by using the same primer set and genomic DNA of strain 168. Incubation of the DNA probe with YetL followed by DNase I digestion was also performed in the presence of 10 mM quercetin or apigenin.
Gel retardation analysis.
Gel retardation analysis was performed essentially as described previously (37). The PyetL and PyetM probes, which were the probes that were used for DNase I footprinting, were labeled by PCR in the presence of [α-32P]dCTP (MP Biomedicals) with the same primer pairs. To create a PyetL probe derivative from which the internal region was deleted, recombinant PCR (8) was performed with the internal overlapping primer pair PyetL_delEF/ PyetL_delER (Table 2) along with the flanking primer pair PyetLF/PyetLR. The labeled probe (0.02 pmol) was mixed and incubated at 30°C for 10 min with various amounts of the YetL protein in a 25-μl reaction mixture, and then the mixture was subjected to PAGE. To evaluate the inhibitory effects of flavonoids on DNA binding of the YetL protein, 1-μl portions of various concentrations of each flavonoid dissolved in DMSO were added to the reaction mixture, which was followed by similar incubation and then electrophoresis.
lacZ fusion analysis to monitor yetL and yetM promoters.
B. subtilis cells were grown in 50 ml of LB medium at 37°C with shaking. When the OD600 reached 0.2, each of the flavonoids dissolved in DMSO was added to the medium to obtain a final concentration of 200 μg/ml, corresponding to concentrations of 0.6, 0.7, 0.7, 0.7, 0.7, 0.7, 0.7, 0.8, 0.7, 0.7, 0.8, and 0.7 mM for quercetin, fisetin, galangin, kaempferol, morin, apigenin, luteolin, chrysin, (+)-catechin, genistein, daidzein, and coumestrol, respectively. As a control, 200 μl of DMSO was added instead of a flavonoid solution. Then 1-ml aliquots of the culture were withdrawn at 1-h intervals, and the β-galactosidase (β-Gal) activity in crude cell extracts was measured spectrophotometrically using o-nitrophenyl-β-d-galactopyranoside (Wako Pure Chemicals Industries, Japan) as a substrate and the procedure described previously (2). To reduce the chromatic disturbance of the β-Gal assay by the flavonoid adhering to the cells, the collected cells were washed with 100 mM phosphate buffer (pH 7.5) before lysozyme treatment.
Flavonoids.
Quercetin, fisetin, kaempferol, morin, apigenin, chrysin, (+)-catechin, genistein, and daidzein were products of Sigma. Galangin was purchased from Extrasynthese S.A., luteolin was purchased from Wako Pure Chemicals Industries, and coumestrol was purchased from Fluka.
RESULTS
DNA microarray analysis to find additional candidate genes induced by flavonoids.
In order to find candidate genes whose expression could be induced by quercetin or fisetin other than the members of the LmrA/YxaF regulon (yxaF, yxaGH, and lmrAB), we performed a DNA microarray analysis to compare the transcriptomes of B. subtilis strain 168 cells grown in the presence and absence of a flavonoid (the DNA microarray data have been deposited in the KEGG Expression Database [http://www.genome.jp/kegg/expression]). As a result, we selected the yetM gene as a candidate, which had not been characterized previously but was predicted to encode an FAD-dependent monooxygenase based on a BLASTP sequence similarity search (http://www.genome.ad.jp/). (A study indicated that quercetin and fisetin are rather weak inducers of yetM expression compared with some other flavonoids, as described below.) Immediately upstream of yetM, the yetL gene encoding a transcriptional regulator belonging to the MarR family (http://bacillus.genome.jp) is in the opposite orientation (Fig. 1). In the framework of the JAFAN (http://bacillus.genome.jp/), a comprehensive DNA microarray analysis of hundreds of putative transcriptional regulators has been conducted, and a DNA microarray analysis involving strains 168 (wild type) and YETLd (a yetL disruptant) indicated that the yetL disruption resulted in a significant increase in yetM transcription (data not shown). Based on all the information, we hypothesize that YetL represses the yetM gene by binding to its cis sequence in the promoter region and that some flavonoids can inhibit DNA binding of YetL to derepress yetM transcription.
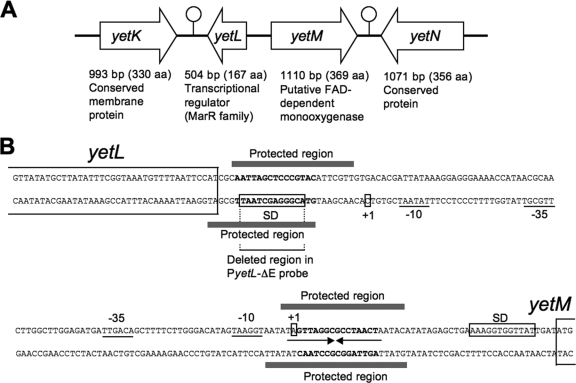
FIG. 1.
Organization of the divergent yetL and yetM genes and their promoter regions. (A) Organization of the yetL and yetM genes. yetL, yetM, and neighboring genes are indicated by large open arrows, and the two hairpin structures that likely function as ρ-independent transcription terminators are indicated by stem-loops structures. aa, amino acids. (B) Promoter regions of the yetL and yetM genes. The “−35” and “−10” sequences of the two genes are underlined, and the transcription start sites (+1) and the SD sequences are enclosed in boxes. The partial coding regions of yetL and yetM are indicated by lines, and the deleted region in PyetL-ΔE is also indicated by a line. The protected regions in the coding and noncoding strands detected by DNase I footprinting are indicated by bars. The 18 bp of a perfect palindromic sequence in the yetM promoter is indicated by a pair of facing arrows, and the sequences conserved between the YetL binding sites of yetL and yetM are indicated by bold type.
Determination of the transcription start sites of the yetL and yetM genes.
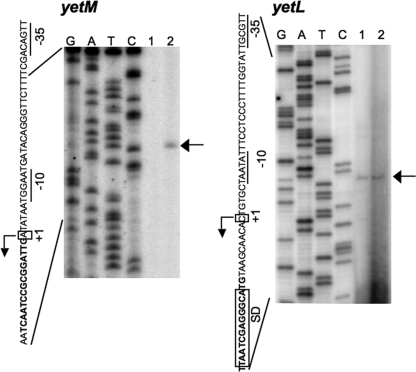
To determine the transcription start site of the yetM gene by primer extension analysis, RNA samples were prepared from cells of strains 168 (wild type) and YETLd (yetL disruptant). As shown in Fig. 2 (left panel), the specific band containing runoff cDNA representing yetM was detected only with the strain YETLd RNA sample, indicating that transcription of yetM is repressed by YetL. This allowed us to identify the transcription initiation site of yetM, and we predicted that the “−35” and “−10” sequences of the yetM promoter are TTGACA and TAAGGT, respectively, with an 18-bp spacer (Fig. 1) and are similar to promoter sequences recognized by σA-RNA polymerase (7).
FIG. 2.
Determination of the transcription start sites of the yetM and yetL genes by primer extension analysis. Total RNAs of strains 168 (wild type) (lane 1) and YETLd (yetL disruptant) (lane 2) used for determination of the transcription start site of yetM (left panel) and total RNAs of FU1035 (yetL+) (lane 1) and FU1038 (ΔyetL::tet) (lane 2) used for determination of the transcription start site of yetL (right panel) were prepared and used for the reverse transcription reaction to generate the runoff cDNAs. Lanes G, A, T, and C contained the products of the dideoxy sequencing reactions obtained with the same primer that was used for reverse transcription. The runoff cDNAs are each indicated by an arrow. The partial nucleotide sequences of the coding strands corresponding to the ladders are shown; the “−35” and “−10” sequences are underlined, and the transcription start sites (+1) and the SD sequence are enclosed in boxes.
To determine the start site of the yetL transcript, we first performed primer extension using RNA samples from strains 168 and YETLd as the templates and the radiolabeled primer specific for the upper part of the yetL ORF. But both the primer extension and DNA sequencing reactions were blocked inside the ORF (data not shown), probably due to blockage of elongation by formation of specific RNA and DNA secondary structures. Then we constructed strains FU1035 and FU1038 without and with the yetL disruption, respectively, in which the yetL promoter fused to the lacZ gene was integrated into the amyE locus (Table 1). Also, we conducted primer extension with a primer specific for lacZ. As shown in Fig. 2 (right panel), the specific band of runoff cDNA was detected with the RNA samples from both strain FU1035 and strain FU1038, but the band derived from the RNA of strain FU1038 seemed to be substantially more intense than the band derived from the RNA of strain FU1035, suggesting that the yetL gene is partially autorepressed. Thus, we determined the transcription start site of yetL and predicted that the “−35” and “−10” sequences of the yetL promoter are TTGCGT and TATAAT with a 17-bp spacer (Fig. 1), which also seems to be recognized by σA-RNA polymerase (7).
Preparation of the YetL protein.
To prepare the YetL protein for in vitro experiments, the yetL gene was cloned in the vector pET-22b(+), and recombinant YetL was overproduced in E. coli BL21(DE3) cells by means of IPTG addition. Purification of YetL almost to homogeneity was achieved by (NH4)2SO4 precipitation followed by anion-exchange column chromatography as described in Materials and Methods. On a sodium dodecyl sulfate-PAGE gel, a single 19.2-kDa protein species was visualized (data not shown). As determined by gel filtration, the YetL protein had a molecular mass of 40.6 kDa (data not shown), indicating that it forms a dimer.
Identification of the binding sites of YetL in the yetL and yetM promoter regions.
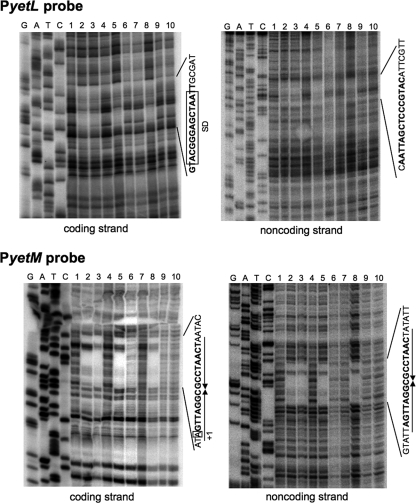
DNase I footprinting analysis was performed to identify each of the YetL binding sites in the yetL and yetM promoter regions. When the YetL protein was mixed with the PyetL probe (bases −68 to 183; base 1 was the transcription start base), YetL protected a region in the yetL promoter against DNase I (bases 11 to 30 of the coding strand and bases 4 to 26 of the noncoding strand) (Fig. 3, upper panels, lanes 2, 3, 5, and 8). The protected sequence overlapped the Shine-Dalgarno (SD) sequence for ribosome binding (Fig. 1). Next, we carried out DNase I footprinting experiments using the PyetM probe (bases −86 to 138; base 1 was the transcription start base). In this analysis, YetL was found to specifically protect its binding site in the yetM promoter region against DNase I (bases −2 to 21 of the coding strand and bases −5 to 21 of the noncoding strand) (Fig. 3, lower panels, lanes 2, 3, 5, and 8), and 18 bp of the complete palindrome sequence was observed (TAGTTAGGCGCCTAACTA; bases −1 to 17) (Fig. 1). These results suggest that YetL binds to the corresponding sites in the yetL and yetM promoter regions to repress their transcription.
FIG. 3.
DNase I footprinting analysis of YetL in the yetL and yetM promoter regions. DNA probes corresponding to the yetL and yetM promoter regions (PyetL and PyetM), 5′ end labeled on either the coding or noncoding strand, were prepared. The 5′-labeled probe (0.8 nM) was incubated in the reaction mixture with the recombinant YetL protein (lanes 2, 5, 6, and 7 for the PyetL probe, 144 nM [a dimer]; lanes 3, 8, 9, and 10 for the PyetL probe, 72 nM; lanes 2, 5, 6, and 7 for the PyetM probe, 36 nM; lanes 3, 8, 9, 10 for the PyetM probe, 18 nM) and without the YetL protein (lanes 1 and 4). A flavonoid solution in DMSO (2 μl) was added to the mixture to obtain a flavonoid concentration of 10 mM (lanes 6 and 9, quercetin; lanes 7 and 10, apigenin), and the same volume of DMSO was added to the mixtures in lanes 5 and 8 before incubation. After partial digestion with DNase I, the resulting mixtures were subjected to urea-PAGE. Lanes G, A, T, and C contained the products of the dideoxy sequencing reactions with the corresponding 5′-labeled primers. Nucleotide sequences protected by YetL are indicated on the right in each panel; the SD sequence and the transcription start site (+1) are enclosed in boxes, the perfect palindrome sequence is indicated by a pair of facing arrows, and the sequences conserved in the protected areas of the PyetL and PyetM probes are indicated by bold type.
Quantitative evaluation of the DNA binding affinity of YetL and its inhibition by various flavonoids by in vitro analysis.
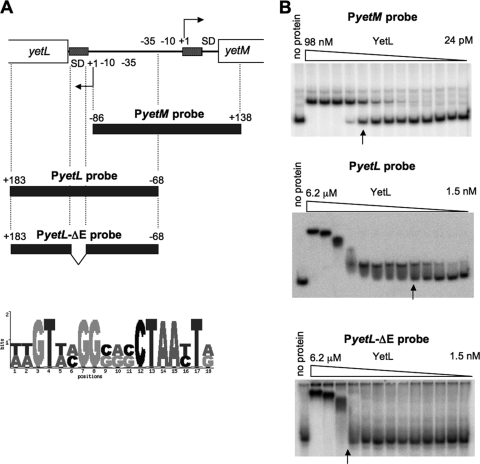
To quantitatively evaluate the YetL binding to the yetL and yetM sites and its inhibition by various flavonoids, we performed gel retardation analysis using the YetL protein and the PyetL and PyetM probes that were used for DNase I footprinting (Fig. 3 and 4A).
FIG. 4.
In vitro binding affinity of YetL to the yetL and yetM cis sequences. (A) Each DNA probe used for gel retardation is indicated by a bar. The YetL-binding cis sequences in the yetL and yetM promoter regions are indicated by cross-hatched boxes. The results of a comparison of these sequences are shown as motif logos created with the B. subtilis Motif Location Search software (http://dbtbs.hgc.jp/motiflocationsearch.html). (B) The 32P-labeled DNA probes for the yetL and yetM promoter regions (PyetL and PyetM) and the 32P-labeled PyetL derivative lacking the cis sequence (PyetL-ΔE) (0.8 nM) were incubated with purified YetL protein, which was followed by PAGE. The YetL protein solution was diluted stepwise twofold, and an aliquot of each dilution was added to the mixture to obtain the concentrations used. The no protein lanes contained no YetL. The arrows indicate lanes for which the Kd was determined. The experiments were repeated at least two times, and the results of representative experiments are shown.
As shown in Fig. 4, YetL bound to each of the PyetL and PyetM probes containing its binding site, which resulted in retarded bands on a PAGE gel depending on the YetL concentration. The binding affinity of YetL for the PyetL probe was weaker than that for the PyetM probe, and the apparent dissociation constants (Kd) of YetL for the PyetL and PyetM probes were estimated to be 24 nM and 6 nM for a dimer, respectively (Fig. 4B). As mentioned above, the YetL binding site for yetM contains a complete 18-bp palindrome sequence, whereas the binding site for yetL contains only a portion of this palindrome but overlaps the SD sequence of yetL. Sequence comparison of the two binding sites revealed only 9 bp that matched in the 18-bp sequences (Fig. 4A). To confirm that the site found to be protected by YetL by DNase I footprinting is actually essential for YetL binding to the yetL promoter, a PyetL probe derivative lacking this site (PyetL-ΔE) was examined for YetL binding affinity. The YetL binding affinity for the PyetL-ΔE probe was found to be remarkably lower (apparent Kd, 1.2 μM) than that for the PyetL probe (24 nM), clearly indicating that this site is indispensable for YetL binding (Fig. 4). There was a second fragment shift from 800 nM to 6.2 μM YetL not only for the PyetL and PyetL-ΔE probes (Fig. 4B) but also for the PyetM probe (data not shown), so the shift appeared to result from nonspecific binding of YetL to the DNA fragment.
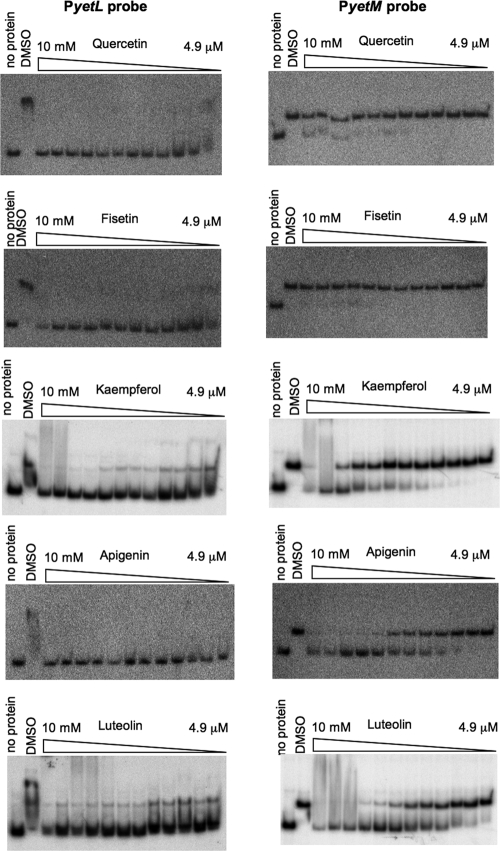
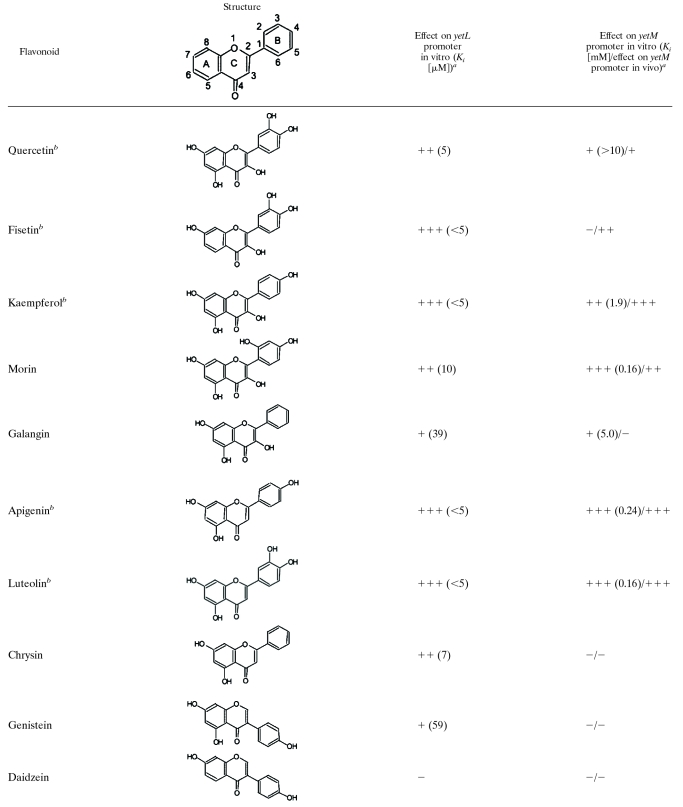
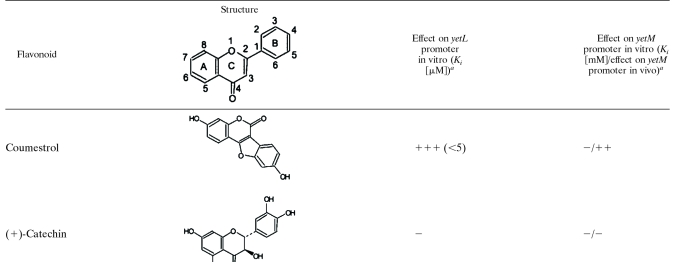
We attempted to quantify the inhibitory effects of various flavonoids on YetL binding to the PyetL and PyetM probes by performing a gel retardation analysis with the YetL concentration fixed at 780 nM and 49 nM for the PyetL and PyetM probes, respectively (Fig. 5); these concentrations were sufficient to cause complete retardation of the probes (Fig. 4B). Five flavonols (quercetin, fisetin, kaempferol, morin, and galangin), three flavones (apigenin, chrysin, and luteolin), three isoflavones (genistein, daidzein, and coumestrol), and (+)-catechin were tested at appropriate concentrations (4.9 μM to 10 mM), and the results are summarized in Table 3; the results for quercetin, fisetin, kaempferol, apigenin, and luteolin are shown in Fig. 5. All of the flavonoids tested except daidzein and (+)-catechin inhibited YetL binding to the PyetL probe, and the inhibitory effects of fisetin, kaempferol, apigenin, luteolin, and coumestrol were prominent (Ki, <5 μM). On the other hand, clear inhibition of YetL binding to the PyetM probe was observed with kaempferol, morin, apigenin, and luteolin (Ki, <2 mM), and there was slight inhibition by quercetin and galangin (Ki, >2 mM). But fisetin, chrysin, genistein, daidzein, coumestrol, and (+)-catechin did not inhibit YetL binding to the PyetM probe even at a concentration of 10 mM. We also tested the inhibitory effects of quercetin and apigenin on YetL binding to the PyetL and PyetM probes using DNase I footprinting. When DNase I digestion was carried out with 10 mM quercetin or apigenin, the specifically protected regions of PyetL and PyetM disappeared (Fig. 3, lanes 6, 7, 9, and 10). The inhibitory effect of quercetin on binding to the PyetM probe was likely so weak that it was detected only by DNase I footprinting.
FIG. 5.
Evaluation of the inhibitory effect of each flavonoid on YetL binding to the yetL and yetM cis sequences by gel retardation. The 32P-labeled PyetL and PyetM probes (0.8 nM) were incubated with 780 nM and 49 nM YetL, respectively, in the presence of each flavonoid indicated at concentrations of 4.9 μM to 10 mM (12 lanes; twofold dilution with DMSO 11 times before incubation). The DMSO lanes contained no flavonoid, and the no protein lanes contained no YetL. The experiments were repeated at least two times, and the results of representative experiments are shown.
TABLE 3.
Structures of flavonoids, their inhibitory effects on YetL binding to the PyetL and PyetM probes, and derepression of the yetM promoter repressed by YetL
aThe data are the results of an evaluation of the in vitro results for the yetL promoter and a comparison of the in vitro and in vivo results for the yetM promoter. The in vitro results were obtained in experiments involving gel retardation, which were performed as described in the legend to Fig. 5, in which the results for addition of quercetin, fisetin, kaempferol, apigenin, or luteolin are shown. Based on the Ki values for the 12 flavonoids for binding of YetL to the PyetL probe, the inhibitory effects are expressed as follows: +++, <5 μM; ++, 5 to 10 μM; +, >10 μM; −, no inhibition. For YetL binding to the PyetM probe, the inhibitory effects are expressed as follows: +++, <0.5 mM; ++, 0.5 to 2 mM; +, >2 mM; −, no inhibition. The actual Ki values are indicated in parentheses. The in vivo results were obtained by performing experiments with lacZ fusions as described in the legend to Fig. 6C, in which the results for addition of quercetin, fisetin, kaempferol, apigenin, or luteolin are shown. Based on the peak β-Gal activity after addition of each flavonoid, the degrees of derepression of the yetM promoter repressed by YetL are expressed as follows; +++, >5 nmol/min per OD600 unit; ++, 1 to 5 nmol/min per OD600 unit; +, <1 nmol/min per OD600 unit; −, no derepression.
Repression of the yetL and yetM promoters by YetL in vivo and release of this repression by certain flavonoids.
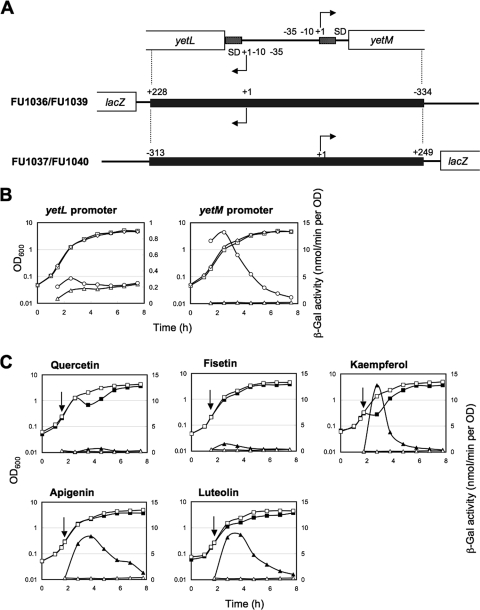
Both DNase I footprinting and gel retardation analyses revealed the YetL binding sites of yetL and yetM, which are likely involved in repression of the promoter activities of these genes. To confirm this in vivo, we constructed two sets of B. subtilis strains without and with the yetL disruption, in which the yetL and yetM promoters fused to the lacZ gene in different orientations were integrated into the amyE locus, respectively. Strains FU1036 (yetL+) and FU1039 (ΔyetL::tet) were used to assess the yetL promoter activity in the presence and absence of YetL, the yetL promoter region (bases −334 to 228), which covers 200 bp of the partial yetL ORF, the entire intergenic region between yetL and yetM, and 200 bp of the partial yetM ORF, being fused to the lacZ gene (Table 1 and Fig. 6A). When the β-Gal activity of each strain was monitored, the activity of strain FU1039 was found to be fairly low but higher than that of strain FU1036, suggesting that YetL represses the yetL promoter activity (Fig. 6B). Then we assessed the yetM promoter activity using strains FU1037 (yetL+) and FU1040 (ΔyetL::tet), the same region that was used for FU1036 and FU1039 (bases −313 to 249 for yetM) being inversely fused so that lacZ was under control of the yetM promoter (Table 1 and Fig. 6A). The β-Gal activity of each strain was monitored, and it was found that the activity of strain FU1040 was always much higher than that of strain FU1037, clearly indicating that YetL represses the yetM promoter activity (Fig. 6B). The derepressed promoter activities of both yetL and yetM gradually decreased as the cultures reached the stationary growth phase, suggesting that these promoters were inactivated during the stationary phase, possibly due to a decrease in RNA polymerase activity associated with σA and/or an unknown regulatory factor(s) other than YetL.
FIG. 6.
Derepression of the yetL and yetM promoter activities repressed by YetL in response to flavonoids. (A) The yetL and yetM promoter regions were fused to the lacZ reporter gene in opposite orientations and then integrated into the amyE loci of strains 168 and FU1034 (ΔyetL::tet) to monitor the yetL and yetM promoters, respectively. In the resulting strains, FU1036 (yetL+) and FU1039 (ΔyetL::tet), the lacZ reporter is under the control of the yetL promoter, whereas in strains FU1037 (yetL+) and FU1040 (ΔyetL::tet), the lacZ reporter is controlled by the yetM promoter. (B) lacZ expression during cultivation of strains FU1036 and FU1037 (left panel) and strains FU1039 and FU1040 (right panel). Squares, OD600 values for strains without the yetL disruption (strains FU1036 and FU1039); diamonds, OD600 values for strains with the yetL disruption (strains FU1039 and FU1040); triangles, β-Gal activities for strains without the yetL disruption (strains FU1036 and FU1037); circles, β-Gal activities for strains with the yetL disruption (strains FU1039 and FU1040). (C) Strain FU1037 was used to monitor the yetM promoter activity in response to addition of each flavonoid. When the OD600 reached 0.2, each flavonoid was added to the culture (indicated by an arrow) at a concentration of 200 μg/ml. The squares and triangles indicate the OD600 values and β-Gal activities, respectively, and the open and filled symbols indicate cultures without and with flavonoids, respectively. The lacZ fusion experiments were repeated at least two times, and the results of representative experiments are shown.
Since each flavonoid had different inhibitory effects on the binding of YetL to the cis sequences of yetL and yetM in vitro, we examined if a flavonoid releases repression of the yetM promoter through the YetL repressor, i.e., if it actually induces the β-Gal activity observed in the lacZ fusion experiments involving strain FU1037. The inducing effects of flavonoids on the yetL promoter were not examined because of the low activity of the intrinsic yetL promoter, as judged in the lacZ fusion experiment involving strain FU1039 (ΔyetL::tet).
The 12 flavonoids examined in the gel retardation analysis were also examined in lacZ fusion experiments, the results of which are summarized in Table 3 together with those obtained in the in vitro analysis. The induction profiles for the β-Gal activity in the presence of quercetin, fisetin, kaempferol, apigenin, and luteolin are shown in Fig. 6C. The β-Gal activity of strain FU1037 increased significantly in the presence of kaempferol, apigenin, and luteolin, and kaempferol was the most effective flavonoid (Table 3 and Fig. 6C). Addition of fisetin, morin, and coumestrol resulted in moderate induction of the β-Gal activity, while addition of quercetin induced β-Gal activity only very slightly (Table 3 and Fig. 6C) and addition of galangin, crysin, genistein, daidzein, and (+)-catechin did not induce β-Gal activity at all (Table 3). These in vivo results essentially agreed with the results of the in vitro gel retardation analysis and indicate that 3 of the 12 flavonoids (kaempferol, apigenin, and luteolin) have significant effects and 3 (fisetin, morin, and coumestrol) have moderate effects as inducers for YetL, the repressor of the yetL and yetM genes, and that they appear to be incorporated (or permeable) in B. subtilis cells.
DISCUSSION
The B. subtilis yetL and yetM genes, which are diversely oriented with respect to each other (Fig. 1), encode a transcriptional regulator belonging to the MarR family and a putative FAD-dependent monooxygenase, respectively. The orientations of the yetL and yetM genes and neighboring genes strongly suggest that yetL and yetM are monocistronic. The transcription initiation bases of the yetL and yetM genes were identified by primer extension analysis (Fig. 2), and the two promoters were likely recognized by RNA polymerase possessing σA.
The DNase I footprinting analysis revealed that YetL binds to the cis sequence in each of the yetL and yetM promoter regions, implying that YetL regulates the expression of these genes separately (Fig. 3). A 18-bp perfect palindrome sequence (TAGTTAGGCGCCTAACTA) was found in the binding site in the yetM promoter region, whereas a perfect palindromic sequence was not found in the binding site in the yetL promoter region (Fig. 1). The YetL protein exhibited much higher affinity for the cis sequence of the yetM promoter region (PyetM probe) than for that of the yetL promoter region (PyetL probe) as determined by gel retardation analysis (Fig. 4B), which might be attributed to the complete palindrome structure in the binding site of yetM. The implication that yetM expression is repressed well by YetL, whereas yetL repression is moderately autoregulated, was confirmed by primer extension analysis (Fig. 2) and the lacZ fusion assay involving the strains without and with the yetL disruption (Fig. 6B).
We tried to find additional members of the YetL regulon by performing DNA microarray analysis involving the wild-type and yetL-deficient strains, as well as a motif search involving the B. subtilis Motif Location Search software (http://dbtbs.hgc.jp/motiflocationsearch.html) and the two YetL binding sequences identified here. However, these approaches were not successful for finding further candidate members of the YetL regulon (data not shown).
To evaluate the inhibitory effects of various flavonoids on the binding of YetL to its cis sequences, a gel retardation analysis with various concentrations of each flavonoid was conducted (Fig. 5 and Table 3). Twelve flavonoids were tested, and all of them except daidzein and (+)-catechin were found to readily release YetL binding to the cis sequence of yetL; the inhibitory effects of fisetin, kaempferol, apigenin, luteolin, and coumestrol were prominent. The inhibitory effects of this broad range of flavonoids were due to the lower affinity of YetL for the yetL cis sequence. On the other hand, the high-affinity binding of YetL to the yetM cis sequence was clearly inhibited by kaempferol, morin, apigenin, and luteolin and slightly inhibited by quercetin and galangin, but no inhibition was observed with the other flavonoids.
The in vivo lacZ fusion experiments showed that several flavonoids were able to induce expression of the lacZ gene placed downstream of the yetM promoter, which supports the in vitro results of the gel retardation analysis described above (i.e., clear induction by kaempferol, apigenin, and luteolin, moderate induction by fisetin, morin, and coumestrol, slight induction by quercetin, and no induction by the other flavonoids) (Fig. 6C and Table 3). Based on these in vitro and in vivo results, we concluded that kaempferol, apigenin, and luteolin certainly act as inducers that release YetL binding to the cis sequence of yetM for derepression of this gene.
To elucidate the structural requirements for flavonoids as inducers of YetL, the inhibitory effects of flavonols (quercetin, fisetin, kaempferol, morin, and galangin) and flavones (apigenin, luteolin, and chrysin) on YetL binding to the yetM cis sequence were compared in vitro and in vivo (Table 3). The flavonol kaempferol and the flavone apigenin with a 4-hydroxyl group on their B-rings were much more effective than the corresponding compounds galangin and crysin without this group (the flavone structure with the ring and carbon assignments is shown in Table 3), suggesting that this group is essential for YetL inhibition. In addition, kaempferol is more effective than quercetin, suggesting that the 3-hydroxyl group on the B-ring of flavonols prevents the interaction with YetL as an inducer. However, when apigenin and luteolin were compared, they were found to be equally effective, which means that the 3-hydroxyl group on the B-ring of flavones does not act adversely. Hence, we suppose that a hydroxyl group at either position 3 of the B-ring or position 3 of the C-ring is permissive but that hydroxyl groups at both positions are nonpermissive. Because the effect of morin is similar to that of kaempferol, it seems that the 2-hydroxyl group on the B-ring does not severely impair the inducer function when a 3-hydroxyl group is on the C-ring. Isoflavones (genistein, daizein, and coumestrol) and (+)-catechin are unlikely to have significant inhibitory effects, implying that the flavone structure might be an essential feature for activity as a YetL inducer.
The specificity of YetL for its inducer flavonoids appears to be distinct from the specificities of the LmrA and YxaF transcriptional regulators described previously (9). While YetL binding to the yetM cis sequence is not as affected by quercetin and fisetin, these flavonols substantially inhibit the binding of LmrA and YxaF to their cis sequences (LmrA/YxaF boxes) (9). Moreover, the inducer specificities of LmrA and YxaF seem somewhat broader than that of YetL. Genistein and coumestrol also affect the binding of both LmrA and YxaF to their boxes, and (+)-catechin exhibits inhibitory activity only for LmrA binding, whereas tamarixetin exhibits inhibitory activity only for YxaF binding. YetL is also distinct from LmrA and YxaF in domain structure. LmrA and YxaF belong to the TetR family of bacterial transcriptional regulatory proteins, which are known to typically possess two functional domains, a highly conserved N-terminal DNA binding domain and a less conserved C-terminal domain involved in both dimerization and effecter binding (21). The crystal structure of the YxaF protein showed that this protein actually has this structural property of this family (25). On the other hand, YetL belongs to the MarR family of bacterial transcriptional regulators. The crystal structures of several MarR family members revealed that they form a dimer structure with a common triangular shape, at the two corners of which winged helix-turn-helix DNA binding motifs are located (1, 5, 10). These DNA binding motifs consist of the internal region of each subunit, and their N and C termini are intertwined with each other to form a core domain. So far, several bacterial transcriptional regulators that recognize and respond to flavonoids have been reported (9, 13, 30). However, to our knowledge, YetL is the first reported member of the MarR family which specifically responds to flavonoids.
The mechanisms underlying signal recognition by members of the MarR family have not been well defined, and whether a common recognition mechanism triggers their derepression remains unclear. It has been reported that two members of the MarR family, B. subtilis OhrR and YodB, sense oxidative thiol stress through oxidative modification of their cysteine residues, which are located at the N terminus of OhrR and the N and C termini of YodB. This modification results in prevention of DNA binding, which is followed by induction of the target genes involved in resistance to oxidizing compounds (10, 16). E. coli MarR, the prototype of the MarR family, can be dissociated from the operator DNA of the marRAB operon, which is involved in multidrug resistance through interaction with a broad range of drugs, including salicylate (5). A high concentration of salicylate was required to obtain the crystal structure of MarR, in which a salicylate molecule was bound to the surface of each of its DNA binding domains (1), suggesting that inducer drugs are able to interfere with the MarR-DNA interaction. The YetL inducers are unlikely to be so reactive that covalent modification within the YetL readily occurs, as is the case for B. subtilis OhrR and YodB, and are also unlikely to directly interrupt the protein-DNA interaction, as is the case for E. coli MarR, because YetL appears to recognize certain flavonoids strictly. We suppose that the flavonoid recognition mechanism of YetL is distinct from those of OhrR, YodB, and MarR. Indeed, we found that YetL binding to the yetM cis sequence is not inhibited by aromatic compounds, such as catechol and salicylate, by means of gel retardation and lacZ fusion analyses (data not shown).
In B. subtilis, the yxaG gene, one of the members of the LmrA/YxaF regulon, encodes quercetin 2,3-dioxygenase, which catalyzes the C-ring cleavage of quercetin, yielding 2-protocatechuoyl-phloroglucinol carboxylic acid and carbon monoxide (4). YxaG exhibits similar dioxygenase activity with several other flavonols (K. Hirooka and Y. Fujita, unpublished results). Thus, it is assumed that flavonols are converted by YxaG to phenolic esters of aromatic carboxylic acids, which could be hydrolyzed to the corresponding aromatic carboxylic compounds by an endogenous esterase with broad substrate specificity in B. subtilis. It has been reported that B. subtilis possesses the yfiE gene, whose expression is induced by catechol and which encodes catechol 2,3-dioxygenase, which converts catechol into 2-hydroxymuconic semialdehyde (29), and the ywhB gene, which encodes 4-oxalocrotonate tautomerase, which catalyzes the interconversion of 2-hydroxymuconate and 4-oxalocrotonate (36). This implies that catechol and its derivatives are degraded through the meta-cleavage pathway via the dehydrogenation route (27). Alternatively, highly electrophilic aromatic compounds, such as catechol and 2-methylhydroquinone, can form S adducts with cellular thiols. These S adducts are assumed to be subjected to thiol-dependent ring cleavage for detoxification by multiple dioxygenase/glyoxalase family enzymes encoded by mhqA, mhqO, and mhqE, which respond to thiol stress and are regulated by MhqR, a MarR-type repressor with an unknown derepression mechanism (32). The yetM gene, identified as the YetL target, is predicted to encode an FAD-dependent monooxygenase containing FAD and NADH binding motifs. The superfamily that contains YetM also includes salicylate monooxygenase that converts salicylate to catechol (15). In addition, it has been reported that an enzyme from an Asteraceae plant species, Chrysanthemum segetum, catalyzes hydroxylation at position 8 of flavonols and flavones using FAD and NADPH as cofactors (6). Thus, we speculate that YetM might catalyze the conversion of the salicylate derivatives derived from the flavonols through YxaG degradation or the direct hydroxylation of flavonols, followed by YxaG degradation, yielding the catechol derivatives, which would flow into the catechol metabolic pathways described above. Our attempts to prepare the recombinant YetM protein in a soluble form were unsuccessful, and there was no difference in growth in the presence of any of the 12 flavonoids tested between the wild-type strain and the strain with yetM disrupted (data not shown); thus, the enzymatic function of YetM remains to be elucidated. Other approaches for characterization of YetM are currently being examined.
Acknowledgments
We are grateful to Y. Moritake, M. Watanabe, R. Yamamoto, K. Kumamoto, and T. Satomura for their help with the experiments. We also thank Kazuo Kobayashi (Nara Institute of Science and Technology, Japan) for providing strain YETLd and for providing the results of a DNA microarray analysis involving this strain, which involved a search for candidate target genes for YetL.
This work was supported by a Grant-in-Aid for Scientific Research on Priority Areas and by a grant from the High-Tech Research Project for Private Universities from the Ministry of Education, Culture, Sports, Science, and Technology of Japan.
Footnotes
Published ahead of print on 27 March 2009.
REFERENCES
- 1.Alekshun, M. N., S. B. Levy, T. R. Mealy, B. A. Seaton, and J. F. Head. 2001. The crystal structure of MarR, a regulator of multiple antibiotic resistance, at 2.3 A resolution. Nat. Struct. Biol. 8710-714. [DOI] [PubMed] [Google Scholar]
- 2.Atkinson, M. R., L. V. Wray, Jr., and S. H. Fisher. 1990. Regulation of histidine and proline degradation enzymes by amino acid availability in Bacillus subtilis. J. Bacteriol. 1724758-4765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bais, H. P., T. L. Weir, L. G. Perry, S. Gilroy, and J. M. Vivanco. 2006. The role of root exudates in rhizosphere interactions with plants and other organisms. Annu. Rev. Plant Biol. 57233-266. [DOI] [PubMed] [Google Scholar]
- 4.Bowater, L., S. A. Fairhurst, V. J. Just, and S. Bornemann. 2004. Bacillus subtilis YxaG is a novel Fe-containing quercetin 2,3-dioxygenase. FEBS Lett. 55745-48. [DOI] [PubMed] [Google Scholar]
- 5.Grkovic, S., M. H. Brown, and R. A. Skurray. 2002. Regulation of bacterial drug export systems. Microbiol. Mol. Biol. Rev. 66671-701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Halbwirth, H., and K. Stich. 2006. An NADPH and FAD dependent enzyme catalyzes hydroxylation of flavonoids in position 8. Phytochemistry 671080-1087. [DOI] [PubMed] [Google Scholar]
- 7.Haldenwang, W. G. 1995. The sigma factors of Bacillus subtilis. Microbiol. Rev. 591-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Higuchi, R. 1990. Recombinant PCR, p. 177-183. In M. A. Innis, D. H. Gelfand, J. J. Sninsky, and T. J. White (ed.), PCR protocols: a guide to methods and applications. Academic Press, San Diego, CA.
- 9.Hirooka, K., S. Kunikane, H. Matsuoka, K. Yoshida, K. Kumamoto, S. Tojo, and Y. Fujita. 2007. Dual regulation of the Bacillus subtilis regulon comprising the lmrAB and yxaGH operons and yxaF gene by two transcriptional repressors, LmrA and YxaF, in response to flavonoids. J. Bacteriol. 1895170-5182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hong, M., M. Fuangthong, J. D. Helmann, and R. G. Brennan. 2005. Structure of an OhrR-ohrA operator complex reveals the DNA binding mechanism of the MarR family. Mol. Cell 20131-141. [DOI] [PubMed] [Google Scholar]
- 11.Horinouchi, S., and B. Weisblum. 1982. Nucleotide sequence and functional map of pC194, a plasmid that specifies inducible chloramphenicol resistance. J. Bacteriol. 150815-825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hund, H. K., J. Breuer, F. Lingens, J. Hüttermann, R. Kappl, and S. Fetzner. 1999. Flavonol 2,4-dioxygenase from Aspergillus niger DSM 821, a type 2 CuII-containing glycoprotein. Eur. J. Biochem. 263871-878. [DOI] [PubMed] [Google Scholar]
- 13.Kobayashi, H., Y. Naciri-Graven, W. J. Broughton, and X. Perret. 2004. Flavonoids induce temporal shifts in gene-expression of nod-box controlled loci in Rhizobium sp. NGR234. Mol. Microbiol. 51335-347. [DOI] [PubMed] [Google Scholar]
- 14.Kobayashi, K., S. D. Ehrlich, A. Albertini, et al. 2003. Essential Bacillus subtilis genes. Proc. Natl. Acad. Sci. USA 1004678-4683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee, J. R., K. R. Min, Y. C. Kim, C. K. Kim, J. Y. Lim, H. J. Yoon, K. H. Min, K. S. Lee, and Y. Kim. 1995. Cloning of the salicylate hydroxylase gene and catechol 2,3-dioxygenase gene, and sequencing of an intergenic sequence between the two genes of Pseudomonas putida KF715. Biochem. Biophys. Res. Commun. 211382-388. [DOI] [PubMed] [Google Scholar]
- 16.Leelakriangsak, M., N. T. T. Huyen, S. Töwe, N. van Duy, D. Becher, M. Hecker, H. Antelmann, and P. Zuber. 2008. Regulation of quinone detoxification by the thiol stress sensing DUF24/MarR-like repressor YodB in Bacillus subtilis. Mol. Microbiol. 671108-1124. [DOI] [PubMed] [Google Scholar]
- 17.Merkens, H., S. Sielker, K. Rose, and S. Fetzner. 2007. A new monocupin quercetinase of Streptomyces sp. FLA: identification and heterologous expression of the queD gene and activity of the recombinant enzyme towards different flavonols. Arch. Microbiol. 187475-487. [DOI] [PubMed] [Google Scholar]
- 18.Miwa, Y., and Y. Fujita. 2001. Involvement of two distinct catabolite-responsive elements in catabolite repression of the Bacillus subtilis myo-inositol (iol) operon. J. Bacteriol. 1835877-5884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Murata, M., S. Ohno, M. Kumano, K. Yamane, and R. Ohki. 2003. Multidrug resistant phenotype of Bacillus subtilis spontaneous mutants isolated in the presence of puromycin and lincomycin. Can. J. Microbiol. 4971-77. [DOI] [PubMed] [Google Scholar]
- 20.Plaper, A., M. Golob, I. Hafner, M. Oblak, T. Solmajer, and R. Jerala. 2003. Characterization of quercetin binding site on DNA gyrase. Biochem. Biophys. Res. Commun. 306530-536. [DOI] [PubMed] [Google Scholar]
- 21.Ramos, J. L., M. Martínez-Bueno, A. J. Molina-Henares, W. Terán, K. Watanabe, X. Zhang, M. T. Gallegos, R. Brennan, and R. Tobes. 2005. The TetR family of transcriptional repressors. Microbiol. Mol. Biol. Rev. 69326-356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rao, J. R., and J. E. Cooper. 1994. Rhizobia catabolize nod gene-inducing flavonoids via C-ring fission mechanisms. J. Bacteriol. 1765409-5413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sambrook, J., and D. W. Russell. 2001. Molecular cloning: a laboratory manual, 3rd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 24.Satomura, T., D. Shimura, K. Asai, Y. Sadaie, K. Hirooka, and Y. Fujita. 2005. Enhancement of glutamine utilization in Bacillus subtilis through the GlnK-GlnL two-component regulatory system. J. Bacteriol. 1874813-4821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Seetharaman, J., D. Kumaran, J. B. Bonanno, S. K. Burley, and S. Swaminathan. 2006. Crystal structure of a putative HTH-type transcriptional regulator yxaF from Bacillus subtilis. Proteins 631087-1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shaw, L. J., P. Morris, and J. E. Hooker. 2006. Perception and modification of plant flavonoid signals by rhizosphere microorganisms. Environ. Microbiol. 81867-1880. [DOI] [PubMed] [Google Scholar]
- 27.Shingler, V., J. Powlowski, and U. Marklund. 1992. Nucleotide sequence and functional analysis of the complete phenol/3,4-dimethylphenol catabolic pathway of Pseudomonas sp. strain CF600. J. Bacteriol. 174711-724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Steinmetz, M., and R. Richter. 1994. Plasmids designed to alter the antibiotic resistance expressed by insertion mutations in Bacillus subtilis, through in vivo recombination. Gene 14279-83. [DOI] [PubMed] [Google Scholar]
- 29.Tam, L. T., C. Eymann, D. Albrecht, R. Sietmann, F. Schauer, M. Hecker, and H. Antelmann. 2006. Differential gene expression in response to phenol and catechol reveals different metabolic activities for the degradation of aromatic compounds in Bacillus subtilis. Environ. Microbiol. 81408-1427. [DOI] [PubMed] [Google Scholar]
- 30.Terán, W., T. Krell, J. L. Ramos, and M. T. Gallegos. 2006. Effector-repressor interactions, binding of a single effector molecule to the operator-bound TtgR homodimer mediates derepression. J. Biol. Chem. 2817102-7109. [DOI] [PubMed] [Google Scholar]
- 31.Tojo, S., T. Satomura, K. Kumamoto, K. Hirooka, and Y. Fujita. 2008. Molecular mechanisms underlying the positive stringent response of the Bacillus subtilis ilv-leu operon, involved in the biosynthesis of branched-chain amino acids. J. Bacteriol. 1906134-6147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Töwe, S., M. Leelakriangsak, K. Kobayashi, N. van Duy, M. Hecker, P. Zuber, and H. Antelmann. 2007. The MarR-type repressor MhqR (YkvE) regulates multiple dioxygenases/glyoxalases and an azoreductase which confer resistance to 2-methylhydroquinone and catechol in Bacillus subtilis. Mol. Microbiol. 6640-54. [DOI] [PubMed] [Google Scholar]
- 33.Ulanowska, K., A. Tkaczyk, G. Konopa, and G. Wegrzyn. 2006. Differential antibacterial activity of genistein arising from global inhibition of DNA, RNA and protein synthesis in some bacterial strains. Arch. Microbiol. 184271-278. [DOI] [PubMed] [Google Scholar]
- 34.Vagner, V., E. Dervyn, and S. D. Ehrlich. 1998. A vector for systematic gene inactivation in Bacillus subtilis. Microbiology 1443097-3104. [DOI] [PubMed] [Google Scholar]
- 35.Wach, A. 1996. PCR-synthesis of marker cassettes with long flanking homology regions for gene disruptions in S. cerevisiae. Yeast 12259-265. [DOI] [PubMed] [Google Scholar]
- 36.Wang, S. C., W. H. Johnson, Jr., R. M. Czerwinski, S. L. Stamps, and C. P. Whitman. 2007. Kinetic and stereochemical analysis of YwhB, a 4-oxalocrotonate tautomerase homologue in Bacillus subtilis: mechanistic implications for the YwhB- and 4-oxalocrotonate tautomerase-catalyzed reactions. Biochemistry 4611919-11929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yoshida, K., Y. Fujita, and A. Sarai. 1993. Missense mutations in the Bacillus subtilis gnt repressor that diminish operator binding ability. J. Mol. Biol. 231167-174. [DOI] [PubMed] [Google Scholar]
- 38.Yoshida, K., I. Ishio, E. Nagakawa, Y. Yamamoto, M. Yamamoto, and Y. Fujita. 2000. Systematic study of gene expression and transcription organization in the gntZ-ywaA region of the Bacillus subtilis genome. Microbiology 146573-579. [DOI] [PubMed] [Google Scholar]
- 39.Yoshida, K., K. Kobayashi, Y. Miwa, C. M. Kang, M. Matsunaga, H. Yamaguchi, S. Tojo, M. Yamamoto, R. Nishi, N. Ogasawara, T. Nakayama, and Y. Fujita. 2001. Combined transcriptome and proteome analysis as a powerful approach to study genes under glucose repression in Bacillus subtilis. Nucleic Acids Res. 29683-692. [DOI] [PMC free article] [PubMed] [Google Scholar]