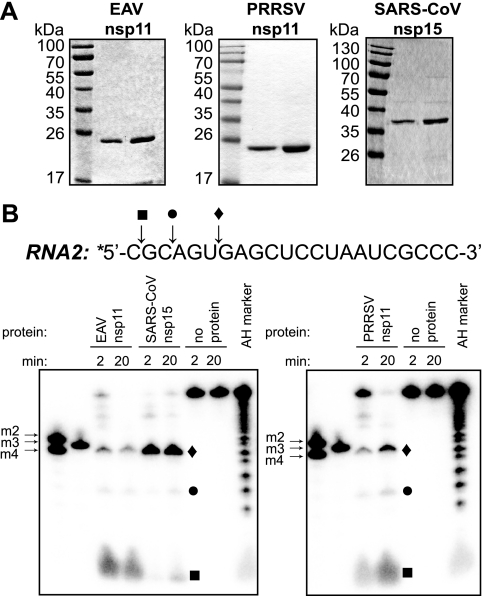
FIG. 2.
Purification and endoribonuclease activity of arterivirus nsp11. (A) Purified recombinant EAV nsp11 (0.5 μg and 1 μg), PRRSV nsp11 (2 μg and 4 μg), and SARS-CoV nsp15 (0.5 μg and 1 μg), each carrying an N-terminal His6 tag, were analyzed by SDS-PAGE and subsequent Coomassie brilliant blue staining. (B) Activity assays using recombinant protein and 2 μM of 5′-32P-labeled (indicated with an asterisk) RNA2 substrate were performed at 30°C for 2 or 20 min. The cleavage products were separated by electrophoresis in a 20% polyacrylamide/7 M urea gel and were visualized by phosphorimager analysis. A nucleotide ladder prepared by alkaline hydrolysis of RNA2 (AH marker), as well as 5′-32P-labeled synthetic short oligoribonucleotides (m2, m3, and m4), were included as size markers. Reactions without enzyme served as negative controls. The sequence of RNA2 is shown above the gel, and cleavage sites 3′ of the cytidylates present in the first (▪) and third (•) positions and 3′ of the uridylate in the sixth position (⧫), and the corresponding cleavage products, are indicated.