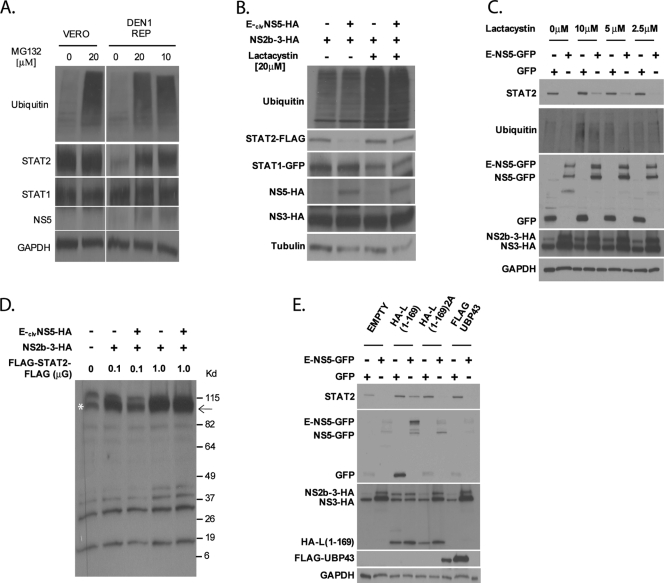
FIG. 5.
Inhibitors of the ubiquitin-proteasome pathway prevent STAT2 degradation by DENV NS5. (A) wtVero or Vero cells stably expressing the DEN1 replicon were treated with the indicated amounts of MG132. Sixteen hours posttreatment, cells were lysed and examined for ubiquitin, STAT2, STAT1, NS5, and GAPDH levels via Western blotting. (B) STAT2-deficient U6A cells were transfected with HA-ubiquitin, STAT2-FLAG, STAT1-GFP, NS2b-3, and either E-clvNS5-HA or an empty vector plasmid. Cells were then treated with lactacystin for 8 h and subsequently lysed and examined by Western blotting using ubiquitin, STAT2, STAT1, HA, and tubulin antibodies. (C) 293T cells were cotransfected with NS2b-3-HA and the plasmids indicated at the top. Ten hours posttransfection, cells were treated with the indicated amounts of lactacystin. Twenty-four hours posttransfection, cells were sorted for GFP-positive cells by FACS, lysed, and examined by Western blotting using ubiquitin-, GFP-, HA-, and GAPDH-specific antibodies. (D) STAT2-deficient U6A cells were transfected with 0.1 or 1 μg of FLAG-STAT2-FLAG, 0.1 μg NS2b-3, and either 1 μg E-clvNS5-HA or an empty vector plasmid. Cells were then lysed and examined by Western blotting using FLAG antibody and long-term film exposure to detect any additional low-intensity bands. The arrow indicates the expected size of FLAG-STAT2-FLAG. The asterisk marks a nonspecific band running at the same mobility of FLAG-STAT2-FLAG. (E) 293T cells were cotransfected with NS2b-3 and the plasmids indicated at the top. Twenty-four hours posttransfection, cells were sorted for GFP-positive cells by FACS, lysed, and examined via Western blotting using STAT2-, GFP-, HA-, FLAG-, and GAPDH-specific antibodies.