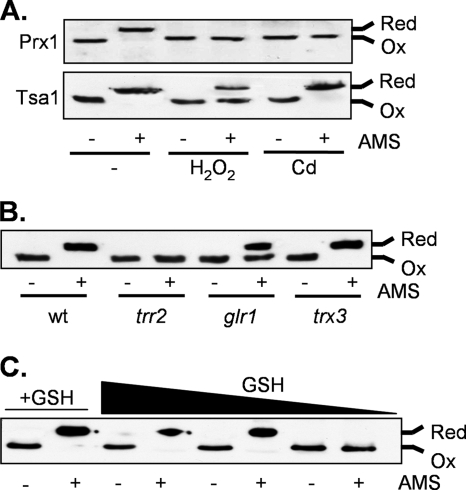
FIG. 5.
Redox state analysis of Prx1. Proteins were precipitated with trichloroacetic acid and free thiols modified by reaction with AMS. Fully oxidized (Ox) and fully reduced (Red) proteins are indicated. (A) Prx1 is oxidized in response to oxidative stress. The wild-type (wt) strain was grown to exponential phase in SD medium and treated with 1 mM hydrogen peroxide or 100 μM cadmium for 1 h. Prx1 and Tsa1 were detected using specific antibodies. (B) The redox state of Prx1 is shifted to a more oxidized form in trr2 and glr1 mutants, but there is no apparent requirement for Trx3. (C) Prx1 becomes oxidized in response to GSH depletion. The gsh1 mutant was grown overnight in YEPD medium and washed with distilled water to remove exogenous GSH. The gsh1 mutant accumulates intracellular GSH from an external medium such as YEPD, which means that the growth of a gsh1 mutant on medium lacking GSH is entirely dependent on the size of the inoculum used, owing to the intracellular GSH accumulated during pregrowth on YEPD medium. The gsh1 mutant was inoculated into SD medium at different initial cellular concentrations (A600 of 0.03, 0.01, and 0.005), and growth continued for 19 h. The black arrow indicates decreasing concentrations of available GSH. As a control, the gsh1 mutant was incubated with 0.1 mM GSH (+GSH) at a starting concentration of 0.001 (lanes 1 and 2).