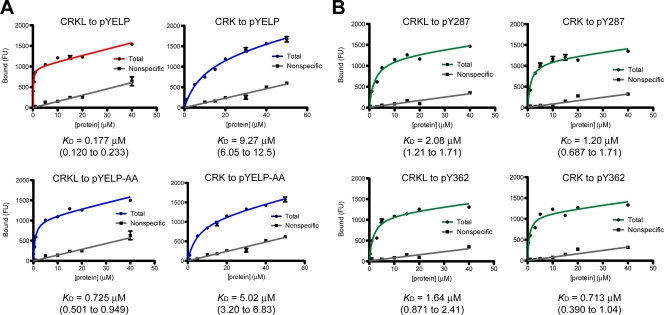
FIG. 3.
Saturation binding experiments for interactions between the SH2 domain of CRKL or CRK and phosphotyrosyl peptides. (A) Representative plots of saturation binding data between the SH2 domain of CRKL or CRK and a peptide corresponding to FGFR1 pY463 (pYELP peptide) or a modified pYELP peptide (pYELP-AA), in which R470/475 are replaced with alanine residues. (B) Representative plots of saturation binding data between CRKL or CRK SH2 domain and a peptide corresponding to BCAR1 pY287 and pY362 peptides. Experiments were carried out using Cy3-labeled SH2 domains after cleavage of GST by thrombin. The x axis shows the concentration of Cy3-labeled SH2 domain, and the y axis shows the arbitrary fluorescent unit (FU) that corresponds to the amount of bound protein. The KD values are shown below the plots. The 95% confidence interval is shown in parentheses.