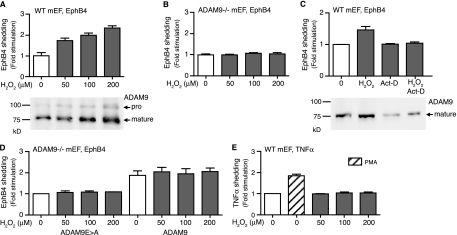
FIG. 6.
ROS increase the levels of expression and activity of ADAM9 in mEFs. (A) Wild-type mEFs (50 to 60% culture density) were treated with 50 to 200 μM of H2O2 for 6 h, and ADAM9 was detected by Western blotting (bottom). Treatment with H2O2 increased the level of expression of ADAM9 in wild-type mEFs. (A and B) Wild-type (A) and Adam9−/− (B) mEFs were transfected with EphB4 as a readout for the catalytic activity of endogenous ADAM9 and treated with increasing concentrations of H2O2. Increased levels of shedding of EphB4 were seen in an H2O2 concentration-dependent manner in wild-type cells (A, top) but not in Adam9−/− cells (B), providing the first evidence for processing of a substrate by endogenously upregulated ADAM9 in wild-type cells. (C) Wild-type (WT) mEFs transfected with EphB4 were either not treated or treated with 200 μM H2O2, with 1 μg/ml actinomycin D (Act-D), or with both 200 μM H2O2 and 1 μg/ml actinomycin D. Actinomycin D prevented the increase in levels of EphB4 shedding from cells treated with H2O2 (top) and the increase in ADAM9 protein levels (bottom), suggesting that H2O2 enhances the transcription of ADAM9. (D) Levels of shedding of EphB4 from Adam9−/− cells can be increased by cotransfection with wild-type ADAM9, but there is no further enhancement by treatment with H2O2 (50 to 200 μM). This suggests that ADAM9 is not directly activated by ROS, since the ADAM9 expression plasmid lacks putative ROS transcriptional regulatory elements. (E) Shedding of TNF-α was used to test whether its sheddase, ADAM17, responds to H2O2 treatment. Phorbol myristate acetate stimulated the shedding of TNF-α in wild-type mEFs, and this is known to depend on ADAM17 (28), whereas increasing concentrations of ROS did not stimulate the shedding of TNF-α in these cells. These results suggest that ADAM9 is the main sheddase responding to ROS treatment for 6 h in mEF cells.