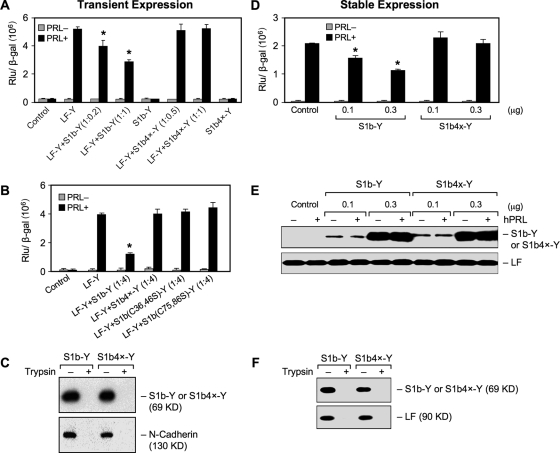
FIG. 1.
(A to C) Functional characterization of a S1b mutant lacking S-S bonds in transient-cotransfection studies. Reporter gene analyses of β-casein-luciferase reporter gene activity in HEK293 cells transfected with a constant hPRLR LF in frame with YFP (Y) (LF-Y; 0.2 μg) with increasing doses of DNA of either the wild-type S1b-Y or S1b4x-Y construct (at the ratio of 1:0.2- to 1-fold [A] of the LF-Y/S1b-Y or S1b4x-Y) or a single-pair cysteine mutant [S1b (C36, 46S)-Y or S1b (C75, 86S)-Y] (1:4-fold; LF-Y:S1b mutant) (B), along with the β-casein-luciferase reporter gene (0.1 μg). The cells were treated with 150 ng/ml hPRL for 16 h before termination. A β-galactosidase (β-Gal) plasmid (0.1 μg) was also included in the transfection, and its activity was measured for normalization of the reporter activity. The highest DNA concentration for the SF (either wild type or mutant) cotransfected with the LF was used in the control S1b-Y- or S1b4x-Y-only group. Empty YFP vector was used as the control and for equalization of DNA transfection. The results were representative of at least three independent experiments (mean plus standard error). The asterisks indicate changes stimulated by hPRL in the group compared to LF-Y with statistical significance (P < 0.01). S1b(Cn, nS), mutation of the cysteine pair to serine occurred at the designated amino acid location in the D1 subdomain; S1b4x, Cys-to-Ser mutation at aa 36, 46, 75, and 86 of the SF S1b. (C) Cell surface expression of wild-type S1b and S1b4x. Biotin-avidin labeling products of transiently expressed PRLR SF (S1b and S1b4x) in HEK293 cells were analyzed by Western blotting using anti-GFP antibody to assess SF expression. The membrane protein marker N-cadherin was used as the positive control to normalize cell surface expression. Normalized cell surface expression of S1b4x-Y relative to S1b was 1.0 ± 0.1 versus 1.14 ± 0.09. Trypsin treatment was used as the negative control. (D to F) Functional characterization of the S1b mutant lacking S-S bonds in HEK293 stably expressing hPRLR LF. HEK293 cells stably expressing the hPRLR LF were transiently transfected with increasing doses of DNA of either wild-type S1b-Y or S1b4x-Y constructs, along with the β-casein-luciferase reporter gene (0.1 μg). The cells were treated with 150 ng/ml hPRL for 16 h before termination. A β-galactosidase plasmid (0.1 μg) was also included in the transfection, and its activity was measured for normalization of the reporter activity (D). Empty YFP vector was used as the control and for equalization of DNA transfection. The results were representative of at least three independent experiments (mean plus standard error). The asterisks indicate changes stimulated by hPRL in the group compared to LF-Y with statistical significance (P < 0.01). (E) Western analysis of endogenous LF and transfected SF (S1b-Y or S1b4x-Y) expression. (F) Cell surface expression of wild-type S1b and S1b4x. Biotin-avidin-labeled products of transiently expressed PRLR SF (S1b and S1b4x) in HEK293 cells stably expressing LF were analyzed by Western blotting using anti-GFP antibody to assess SF expression. Cells stably expressing LF were used as the positive control to normalize cell surface expression. The normalized cell surface expression of S1b4x-Y relative to S1b was 1.0 ± 0.1 versus 0.82 ± 0.09. Trypsin treatment was used as a negative control.