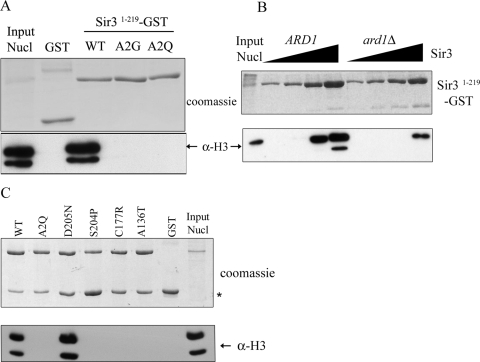
FIG. 6.
Sir3 BAH domain binds to nucleosomes. (A) The N terminus of Sir3 is important for binding to nucleosomes. Wild-type (W303-1a) nucleosomes were mixed with GST, Sir31-219-GST, Sir3A2G 1-219-GST, or Sir3A2Q 1-219-GST bound to glutathione-Sepharose beads. One-third of the nucleosomes retained by the GST-tagged protein on the beads was visualized by Western blotting with anti-H3 antibody. “Input Nucl” refers to 1% of nucleosomes used for the binding reaction. (B) N-terminal acetylation of Sir3 stimulates binding to nucleosomes. Wild-type (W303-1a) nucleosomes were incubated with increasing concentrations of wild-type Sir31-219-GST purified from ARD1 (W303-1a) and ard1Δ (JCY5) strains. One-third of the nucleosomes retained on the beads was visualized as described for panel A. (C) Loss-of-function Sir3 BAH domain mutants are unable to bind to nucleosomes, whereas the D205N mutant binds well. Nucleosomes from W303-1a were incubated with wild-type Sir31-219-GST purified from E. coli and with the indicated Sir3 mutants. One-fourth of the nucleosomes retained on the beads was visualized as described for panel A. “Input Nucl” refers to 0.5% of nucleosomes used for the binding reaction. The asterisk indicates the position of a Sir3-GST degradation product. WT, wild type; α, anti.