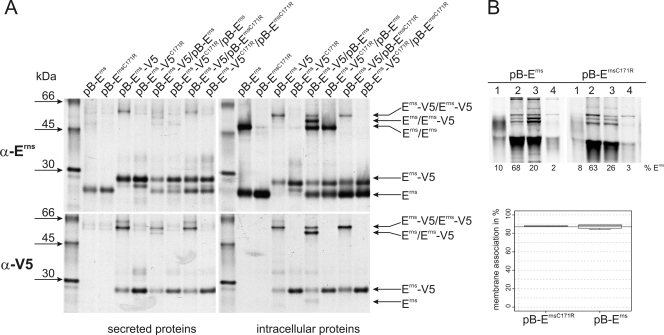
FIG. 1.
(A) Coprecipitation experiments. Tagged (pB-Erns-V5 and pB-Erns-V5C171R) and untagged (pB-Erns and pB-Erns-C171R) variants of Erns from BVDV CP7 with or without C171 were (co)expressed in BHK-21 cells and metabolically labeled. Cells were lysed with a lysis buffer containing 1% Triton X-100 and proteins precipitated from the lysate (right panels) or the cell culture supernatant (left panels) with an Erns-specific serum (α-Erns, upper panels) or a V5 specific antibody (α-V5, lower panels) and deglycosylated before separation through SDS-PAGE under nonreducing conditions. The transfected plasmids or plasmid combinations are indicated at the top. The positions in the gel of the tagged and untagged monomers as well as the positions of the different dimers are indicated by arrows. The antisera used for precipitation are indicated on the left, together with the molecular masses of the size marker bands. (B) Determination of membrane binding/secretion of wt and mutant Erns. The upper part shows an example of SDS-PAGE under reducing conditions of the proteins precipitated from different fractions of cells transfected with expression plasmids given above the respective lanes. 1, secreted proteins; 2, debris; 3, membrane fraction; 4, soluble proteins. The lower panel shows for each construct the calculated level of membrane association in percent. Both constructs were tested at least three times, and the meridian, upper, and lower percentiles are shown. The wt membrane association is indicated by the horizontal line (mean of wt membrane association). The mutated protein was judged to have no significantly different membrane association compared to the wt Erns using the Welch test for unequal variances.