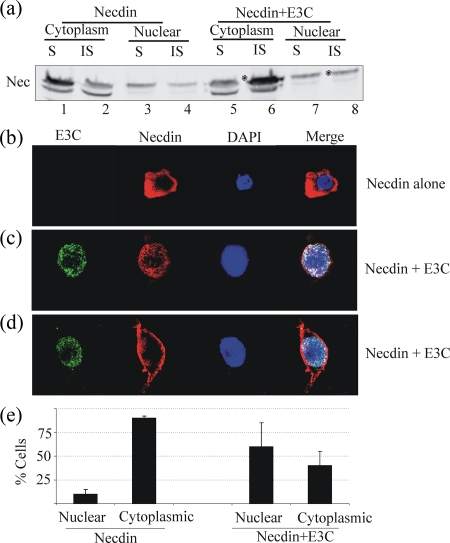
FIG. 2.
(a) The majority of Necdin associates with the insoluble membrane fraction (asterisks) of cytoplasmic (compare lanes 2 and 6) or nuclear (compare lanes 4 and 8) extracts in the presence of EBNA3C. The cytoplasmic soluble fraction had a lower proportion of Necdin (lanes 1 and 5), whereas not much change was seen in the nuclear soluble fraction (lanes 3 and 7). S, soluble; IS, insoluble. (b) Immunofluorescence experiments were performed to visualize the localization of Necdin and EBNA3C. The subcellular localization of Necdin is altered in the presence of EBNA3C. Transfected HeLa cells were overlaid on coverslips and allowed to grow for 48 h. Cells were then washed with PBS, fixed with 1:1 methanol-acetone for 10 min at −20°C, dried, and rehydrated with PBS. For blocking, cells were incubated with PBS containing 3% bovine serum albumin and 1% glycine for 30 min. Cells were then incubated with appropriate antibodies (a 1:100 dilution of anti-Myc antibody and a 1:100 dilution of anti-HA antibody). Slides were washed three times in PBS and further incubated with a 1:1,000 dilution of goat anti-mouse or goat anti-human immunoglobulin fluorescein isothiocyanate-conjugated secondary antibodies. Slides were examined with a confocal microscope and images captured. The cells which did not show any Necdin signal in the nucleus were classified as cytoplasmic. Those cells which also showed a Necdin signal in nucleus were classified as those in which a change in localization had occurred. Necdin localizes primarily in the cytoplasm in most cells. (c and d) Necdin localization is altered in the presence of EBNA3C (c) and occurs in insoluble fractions probably complexing with membranous proteins (d). (d and e) Analysis of 100 cells for Necdin staining showed that changes in localization of Necdin to membranes and nucleus were seen in about 60% cells when they were coexpressing EBNA3C. Error bars indicate standard deviations.