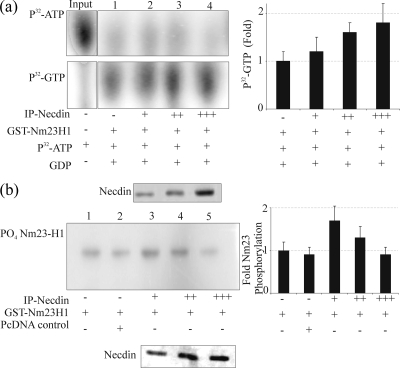
FIG. 4.
(a) The NDP kinase activity of Nm23H1 is increased in the presence of Necdin. Three micrograms of GST-Nm23-H1 with increasing amounts of immunoprecipitated Necdin was incubated with 1 μM [γ-32P]ATP (0.2 mCi/ml) and 100 μM GDP in TMD buffer in a final volume of 20 μl for 10 min at room temperature. The reaction was stopped by the addition of 20 μl of 50 mM EDTA (pH 8.0). The reaction mixture (2 μl) was spotted onto 20- by 20-cm polyethyleneimine-cellulose TLC plates (45), and reaction products were resolved by capillary action in 0.75 M KH2PO4, pH 3.65. The TLC plates were dried, exposed to PhosphorImager plates (Molecular Dynamics, Inc.), and analyzed. The top panel shows [γ-32P]ATP. The amount of [γ-32P]ATP used in each reaction is shown in the input lane. Lanes 1 to 4 (upper panel) show the unused [γ-32P]ATP, and lanes 1 to 4 in lower panel show the amount of [γ-32P]GTP synthesized. The right panel shows the quantification of [γ-32P]GTP as averages from three separate experiments. (b) Autophosphorylation activity of Nm23H1 is reduced in the presence of Necdin. A GST fusion protein of Nm23-H1 was incubated in the absence or presence of increasing amounts of immunoprecipitated Necdin. The reaction was initiated by the addition of 5 μCi of [γ-32P]ATP. After 5 min at 25°C, the reaction was stopped with Laemmli buffer; the samples were incubated for an additional 10 min and were subjected to 12% SDS-PAGE with and without boiling. The procedure without boiling was used to preserve the phosphorylation of Nm23 on the histidine residues. The gels were dried, exposed to PhosphorImager plates, and analyzed. In the left panel, lanes 1 and 2 represent the controls, and lanes 3, 4, and 5 show levels of Nm23-H1 autophosphorylation. The quantitation of autophosphorylation levels is shown in the right panel. Error bars indicate standard deviations.