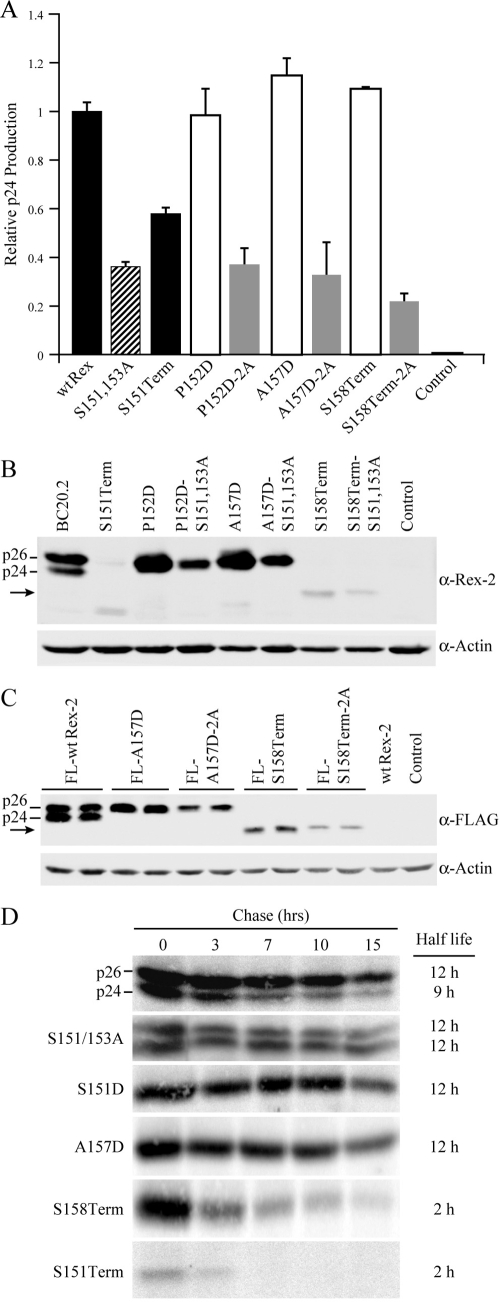
FIG. 3.
The C terminus is crucial for Rex protein expression and stability. (A) Disruption of phosphorylation at serines 151 and 153 (by deletion or alanine substitution mutation) consistently impairs Rex functional activity. 293T cells were transfected with 0.25 μg pcTat, 0.5 μg pcGagRxRE-II, 0.05 μg CMV-Luc, and 0.5 μg wt Rex or mutant Rex plasmids as indicated. Forty-eight hours after transfection, cells were harvested and assayed for p24 Gag. The values, which are normalized and shown relative to wt Rex-2, represent relative p24 Gag production from a representative experiment performed in triplicate. Error bars indicate standard deviations. (B) Rex-2 proteins expressed from transiently transfected 293T cells were detected by Western blot analysis using anti-Rex-2 specific antisera. wt p24 and p26 are indicated, and the arrow identifies the truncated Rex. Detection of cellular β-actin was used as a loading control. (C) The altered expression levels of Rex-2 mutants are not attributable to the detection sensitivity of our anti-Rex-2 antisera. Flag-tagged Rex protein expression (the FLAG-wt Rex, FLAG-RexA157D, FLAG-RexA157D-2A, FLAG-RexS158Term, and FLAG-RexS158Term-2A mutants) was detected from transiently transfected 293T cells using anti-FLAG monoclonal antibody M2. wt p24 and p26 are indicated, and the arrow identifies the truncated Rex. (D) The half-lives of wt Rex, S151/S153A, S151D, A157D, S158Term, and S151Term mutants were determined by pulse-chase experiments as described in Materials and Methods. Quantification of protein at different time points using the Typhoon imaging system was utilized to determine the protein half-life. α, anti.