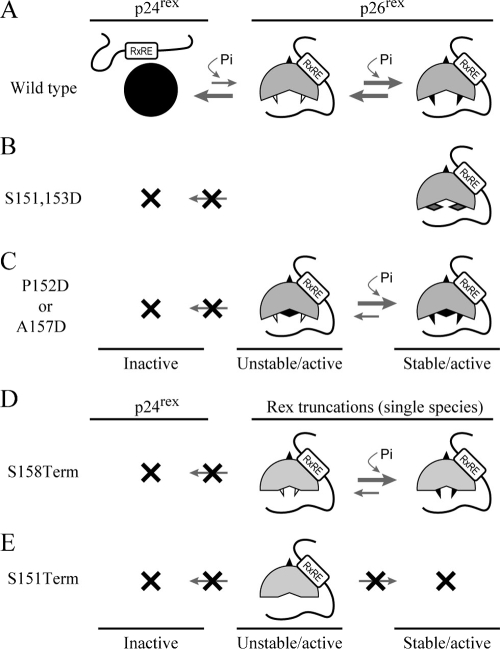
FIG. 7.
Model for Rex-2 phosphorylation and functional regulation. (A) The primary Rex-2 translation product p24 is inactive. An initial phosphorylation on an unidentified serine(s) induces a conformational alteration and results in an unstable but functionally active p26 intermediate. This intermediate can be further stabilized by subsequent phosphorylation on serine 151 and/or 153, generating a fully functional, stable p26 form. “Pi” represents phosphorylation, open triangles denote nonphosphorylated serines, and filled triangles denote phosphorylated serines. (B) The Rex S151 and 153D mutant disrupts the equilibrium between inactive p24rex and active p26rex because the aspartic acids (solid diamonds) are not subjected to dephosphorylation. (C) In Rex P152D and A157D mutants, introduction of a phosphomimetic aspartic acid (solid diamond) into the carboxy terminus functionally overrides the initial phosphorylation on an unidentified serine(s), removes the inhibitory effect of the carboxy terminus, and results in an unstable p26rex active form. Rex-2 is locked in the p26rex form because the aspartic amino acid is not subjected to dephosphorylation. (D) Deletion of the sequence downstream of Ser158 permanently removes the inhibitory carboxy terminus and interrupts the equilibrium between the p24rex inactive form and the p26rex active form. The p26rex intermediate can be stabilized by phosphorylation on serine 151 and/or 153, whereas Rex expressed from the S151Term mutant (E) is conformationally unstable because serines 151 and 153 are deleted.