Abstract
The programmed degradation of macromolecules during petal senescence allows the plant to remobilize nutrients from dying to developing tissues. Ethylene is involved in regulating the timing of nucleic acid degradation in petunia, but it is not clear if ethylene has a role in the remobilization of phosphorus during petal senescence. To investigate ethylene's role in nutrient remobilization, the P content of petals (collectively called the corolla) during early development and senescence was compared in ethylene-sensitive wild type Petunia×hybrida ‘Mitchell Diploid’ (MD) and transgenic petunias with reduced sensitivity to ethylene (35S::etr1-1). When compared to the total P content of corollas on the day of flower opening (the early non-senescing stage), P in MD corollas had decreased 74% by the late stage of senescence (advanced wilting). By contrast, P levels were only reduced by an average of 32% during etr1-1 corolla (lines 44568 and Z00-35-10) senescence. A high-affinity phosphate transporter, PhPT1 (PhPht1;1), was cloned from senescing petunia corollas by RT-PCR. PhPT1 expression was up-regulated during MD corolla senescence and a much smaller increase was detected during the senescence of etr1-1 petunia corollas. PhPT1 mRNA levels showed a rapid increase in detached corollas (treated at 1 d after flower opening) following treatment with low levels of ethylene (0.1 μl l-1). Transcripts accumulated in the presence of the protein synthesis inhibitor, cycloheximide, indicating that PhPT1 is a primary ethylene response gene. PhPT1 is a putative phosphate transporter that may function in Pi translocation during senescence.
Keywords: Ethylene, flowers, high-affinity phosphate transporter, hormones, nitrogen, petal senescence
Introduction
Many flowers have large, showy petals that serve to attract pollinators. The maintenance of petals is costly in terms of respiratory energy, nutrients, and water loss (Stead et al., 2006). Once the flower has been pollinated or the stigma is no longer receptive to pollination, the petals (collectively called the corolla) undergo senescence. A genetically controlled senescence programme allows the plant to dismantle macromolecules and organelles from dying corollas and to remobilize essential nutrients to developing tissues (Jones, 2004; Stead et al., 2006).
Changes in the mineral nutrient content of petunia corollas indicate that nitrogen, phosphorus, and potassium are remobilized during the natural senescence of unpollinated flowers (Verlinden, 2003). Corolla P levels decline by 75%, while the N and K content decreases by only 50% and 40%, respectively (Verlinden, 2003). Additional nutrients, including chromium, copper, iron, molybdenum, sulphur, and zinc are remobilized during the senescence of Arabidopsis leaves (Himelblau and Amasino, 2001). While carbon levels decrease during both petal and leaf senescence, it is unclear whether this is the result of C recycling or tissue respiration (Himelblau and Amasino, 2001; Verlinden, 2003). When compared to newly expanded leaves, petals have much lower levels of both macro and micronutrients. These differences may explain why energy is expended to recycle only the most essential macronutrients from transient tissues like the petals (Verlinden, 2003).
Petal senescence in many species, including petunia, is regulated by the plant hormone ethylene (Woltering and van Doorn, 1988; Borochov and Woodson, 1989). Transgenic petunias with reduced sensitivity to ethylene have been generated by constitutively over-expressing the mutated ethylene receptor from Arabidopsis (35S::etr1-1; Wilkinson et al., 1997). These petunias serve as a good model for investigating the regulation of senescence-specific enzyme activity and the control of gene expression by ethylene. Flower senescence in etr1-1 petunias is delayed by 8–12 d depending on temperature and other growing conditions (Gubrium et al., 2000; Jones et al., 2005; Langston et al., 2005). Induction of the senescence-specific endonuclease, PhNUC1, is also delayed in etr1-1 petunia flowers, and activity is first detected when the corollas are showing visual symptoms of wilting (Langston et al., 2005). Similarly, the up-regulation of five of the six senescence-enhanced cysteine protease genes recently identified from petunia also coincides with the delayed senescence of etr1-1 corollas (Jones et al., 2005). These studies indicate that ethylene is involved in regulating the timing of nucleic acid and protein degradation during petal senescence, but it is not clear if ethylene has a role in the remobilization of nutrients following macromolecule degradation.
While many genes putatively involved in macromolecule and organelle degradation have been identified in screens for senescence-enhanced genes (Buchanan-Wollaston et al., 2003; Jones, 2004; Stead et al., 2006), comparatively little is known about the genes whose products facilitate nutrient remobilization from senescing tissues. Following the catabolism of proteins, nitrogen is exported from senescing tissues via the phloem in the form of the amino acids glutamine and asparagine (Kamachi et al., 1992). Both glutamine synthetase and asparagine synthetase genes, whose protein products catalyse the conversion of ammonia to glutamine and asparagine, respectively, have been identified in senescing leaves and petals, supporting their role in N remobilization (Buchanan-Wollaston and Ainsworth, 1997; Eason et al., 2000). Recent large-scale expression profiling in Arabidopsis has identified a number of transport proteins, including phosphate transporters, which are up-regulated during natural leaf senescence (van der Graaff et al., 2006). By contrast, genes encoding phosphate transporter proteins have not been specifically reported in screens for senescence-enhanced genes in petals.
Phosphorus is an essential macronutrient, which is required for the synthesis of nucleic acids, phospholipids, and cellular metabolites including energy-providing ATP. Phosphate (Pi) is the least available nutrient in the soil and plants have evolved phosphate starvation response mechanisms that allow them to survive under phosphate-limiting conditions (Raghothama and Karthikeyan, 2005). One of these responses is the activation of high-affinity Pi transport, which results in enhanced Pi acquisition from the soil. Genes encoding high-affinity phosphate transporters have been identified from a number of species, and most of these genes have been found to be expressed in roots and to be induced by phosphate starvation (Mudge et al., 2002; Bucher, 2007). Five putative high-affinity phosphate transporters (PhPT1–PhPT5) were recently cloned from Petunia×hybrida to study the role of symbiotic Pi transport in mycorrhizal roots (Wegmuller et al., 2008). PhPT expression was investigated in roots in the absence and presence of arbuscular mycorrhizae, but expression was not investigated in vegetative or reproductive tissues (Wegmuller et al., 2008). The expression of some high-affinity phosphate transporters has been reported in flowers and senescing leaves, suggesting that phosphate transporters may play a role in Pi translocation or remobilization within the plant as well as Pi acquisition (Baek et al., 2001; Kai et al., 2002; Karthikeyan et al., 2002; Mudge et al., 2002; Rae et al., 2003; Chen et al., 2007). While expression studies have indicated a putative role for phosphate transporters in Pi translocation within the plant, the role of phosphate transporters in Pi remobilization during petal senescence has not been investigated.
The main objectives of this research were to determine if ethylene signalling affects the P content of senescing petals and to determine if high-affinity phosphate transporters are expressed during corolla senescence. A high-affinity phosphate transporter, PhPT1 (PhPht1;1), was cloned from senescing petunia corollas and its expression during senescence and following ethylene treatment was investigated. The role of ethylene signalling in Pi reallocation during petal senescence is discussed.
Materials and methods
Plant materials
Petunia×hybrida ‘Mitchell Diploid’ (MD) plants were used in all experiments unless otherwise stated. Comparative analyses using MD plants transformed with 35S::etr1-1 (etr1-1; lines Z00-35-10 and 44568) were conducted to determine the role of ethylene in nutrient remobilization and phosphate transporter gene expression. Etr1-1 seeds were obtained from Dr David Clark (University of Florida). Seeds were treated with 100 mg l-1 GA3 for 24 h and sown in cell-packs on top of soil-less mix (Promix BX, Premier Horticulture, Quebec, Canada). All plants were established in the greenhouse after germination and plants were transferred to 16 cm pots after 4 weeks. Plants were fertilized at each watering with 150 mg l-1 Scott's Excel 15N–5P–15K (The Scotts Co., Marysville, OH). A one-time treatment of Soluble Trace Element Mix (S.T.E.M., The Scotts Co., Marysville, OH) was applied 4 weeks after transferring to 16 cm pots. Growing conditions were 24/16 °C (day/night) with a 13 h photoperiod supplemented by high pressure sodium and metal halide lights.
Nutrient analysis
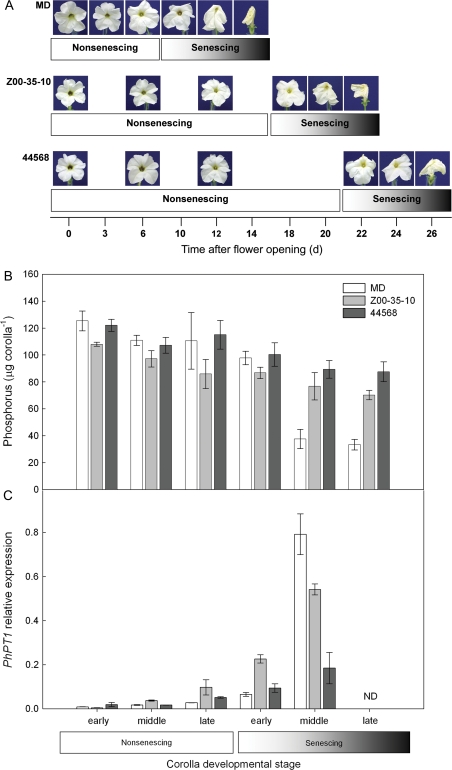
Flowers were emasculated 1 d before flower opening to prevent self-pollination. Corollas were collected from MD and etr1-1 lines (44568 and Z00-35-10) at various times after flower opening. Collection times for nutrient analyses were determined by the flower longevity of each line and represented early, middle, and late stages of non-senescing flowers and early, middle, and late stages of senescing flowers (Fig. 1A). The exact flower ages (number of days after flower opening) that correspond to the developmental stages are shown in Fig. 1 for all three petunia genotypes.
Fig. 1.
Ethylene signalling affects total phosphorus changes and PhPT1 expression during petunia corolla senescence. (A) Natural senescence of unpollinated corollas from Petunia×hybrida ‘Mitchell Diploid’ (MD) wild-type plants and transgenic petunias with reduced sensitivity to ethylene (35S::etr1-1; lines Z00-35-10 and 44568). Flower longevity varied among MD and etr1-1 lines, therefore corollas were collected for nutrient and gene expression analyses at three stages of development (non-senescing—early, middle, and late) and three stages of senescence (senescing—early, middle, and late). (B) Corolla phosphorus content (mean ±SD, n=3) in MD and etr1-1 transgenic lines (Z00-35-10 and 44568) during flower development and senescence. (C) PhPT1 transcript levels (mean ±SD, n=3) in corollas. Relative mRNA abundance compared to PhACTIN was determined by quantitative RT-PCR. ND indicates expression levels were not determined.
Three sets of corollas, containing 21 corollas each, were collected for each time point. Six corollas from each set were frozen in liquid N2 and stored at –80 °C for RNA extraction. The remaining corollas were dried at 60 °C for 3 d for nutrient analysis. The dried corollas were then ground to pass through a 2 mm sieve. All nutrient analyses were conducted at the Service Testing and Research Laboratory (The Ohio State University/OARDC, Wooster, OH). Total nitrogen analysis was conducted on a 100 mg DW sample using the Dumas combustion method (Vario Max combustion analyser; Elementar America, Inc.; Germany) (Sweeney, 1989). Following perchloric/nitric acid digestion, a 250 mg DW sample was analysed for phosphorus and potassium using an inductively coupled plasma spectrometer (ICP) (model PS3000, Leeman Labs Inc., Hudson, NH) as described in Isaac and Johnson (1985). All data are presented as the amount of an individual nutrient per corolla.
Cloning of a petunia phosphate transporter gene
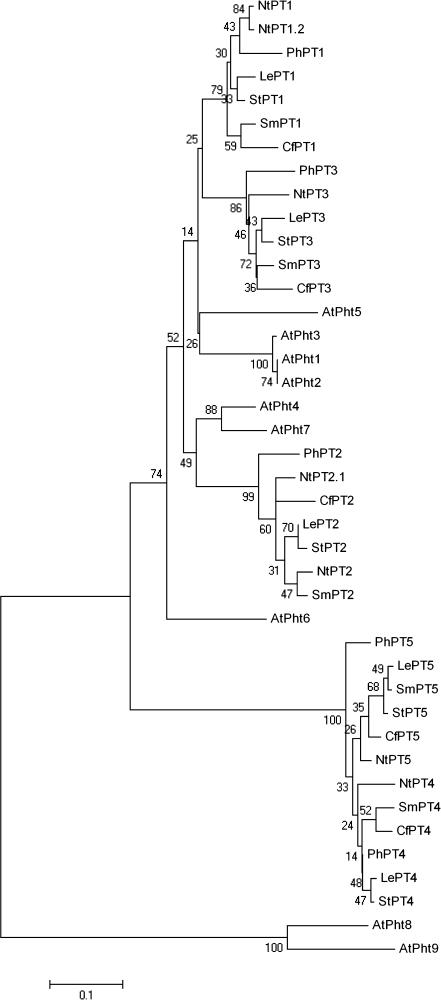
RT-PCR was used to clone a putative high-affinity phosphate transporter from petunia petals. Total RNA was extracted from senescing MD corollas at 72 h after pollination using TRIzol reagent (Invitrogen, Carlsbad, CA) and first-strand cDNA was synthesized using the Omniscript Reverse Transcriptase kit (Qiagen, Valencia, CA). Specific primers were designed based on conserved sequences in the Pht1 cDNAs from tomato and potato. These included the forward primer 5′-GGCGTATGAAGATGCCTGAAAC-3′ and the reverse primer 5′-GCCTGGCTGGGAAAATCTC-3′. Using 2 μg of first-strand cDNA as the template, a petunia phosphate transporter, PhPT1, was amplified with MasterTaq polymerase (Eppendorf, New York). The remaining 5′ and 3′ cDNA sequences were isolated by rapid amplification of cDNA ends (RACE) (SMART RACE kit, Clontech, Mountain View, CA). The full-length PhPT1 cDNA was then isolated by RT-PCR, using the forward primer 5′-ATGGCTAAAGATTTGCAAGTGC-3′ and reverse primer 5′-TTAAACTGGAACAGTCCTTCCA-3′ (PhPT1 GenBank Accession no. EF564180). Sequence information for the full-length cDNA was obtained by capillary sequencing at the Molecular and Cellular Imaging Center (The Ohio State University/OARDC, Wooster, OH) and analysed with ChromasPro and with the BLAST algorithm from the NCBI non-redundant database. The Neighbor–Joining tree of high-affinity (Pht1) phosphate transporters was constructed by the MEGA 4.0 software with bootstrap values 0-100 (Tamura et al., 2007). Clustal 2.0.10 was used for multiple amino acid sequence alignments (Larkin et al., 2007) and TopPred II was used to predict the membrane spanning regions (Claros and von Heijne, 1994).
Ethylene and cycloheximide treatments
Six flowers were removed from MD plants 1 d after flower opening and placed in vials of water. Flowers were then sealed in 24 l chambers and treated with air (control; 0 μl l-1) or 0.1 μl l-1 ethylene for 0.5, 1, 2, or 4 h. MD and etr1-1 44568 flowers were also placed in water or 50 μM cycloheximide and treated with air or 0.1 μl l-1 ethylene for 4 h. Eight-week-old MD, Z00-35-10, and 44568 plants were sealed in chambers and treated with 10 μl l-1 for 4 h. Immediately following ethylene treatments, corolla or leaf tissue was frozen in liquid N2 and stored at –80 °C until needed for RNA extraction.
Expression analysis
Real-time RT-PCR analysis was used to characterize the expression of five petunia phosphate transporter genes during development and following ethylene treatment. These included the senescence-related phosphate transporter (PhPT1) identified in this study and four additional phosphate transporters identified from GenBank (GenBank Accession nos EU532761–EU532764). Total RNA was extracted from petunia corollas using TRIzol reagent (Invitrogen, Carlsbad, CA) and RNA was treated with RQ1 RNase-free DNase (Promega, Madison, WI). cDNA was synthesized from 2 μg RNA using the Omniscript Reverse Transcriptase kit (Qiagen, Valencia, CA). Quantitative PCR was performed in a 20 μl reaction volume using iQ SYBR Green Master Mix (Bio-Rad, Hercules, CA). One microlitre cDNA was used as the template, and all reactions were preformed in triplicate. PCR was conducted for 40 cycles of 94 °C for 10 s, 65 °C for 30 s, 72 °C for 30 s using the iQ5 Thermocycler (Bio-Rad, Hercules, CA). Primers were designed to amplify transcripts from the five petunia phosphate transporters using IDT Primer Quest (see Supplementary Table S1 at JXB online). Melt curves were generated to check amplification specificity and relative target gene expression was normalized to PhACTIN expression for each cDNA sample.
Statistical analysis
Analysis of variance (ANOVA) was conducted using the general linear model procedure in the SAS software (Version 9.0, SAS Institute Inc., Cary, NC, USA).
Results
Corolla senescence is delayed in etr1-1 flowers, but senescence symptoms are similar to MD flowers
Transgenic petunias with reduced ethylene sensitivity have been generated by ectopically expressing the mutant ethylene receptor gene etr1-1 from Arabidopsis (Wilkinson et al., 1997). Independent transgenic lines have a moderate to strong reduction in ethylene sensitivity (Shibuya et al., 2004). Two of these etr1-1 transgenic lines have been used in comparative studies with non-transformed Petunia×hybrida ‘Mitchell Diploid’ (MD) to determine the role of ethylene in Pi remobilization during petal senescence.
Etr1-1 flower senescence was delayed by 8–12 d in lines Z00-35-10 and 44568, respectively, compared to MD (Fig. 1A). The main visual symptom of senescence was corolla wilting. Corollas for comparative analysis between MD and etr1-1 were collected from three stages of non-senescing and three stages of senescing flowers as indicated in Fig. 1. The early stage of senescence was characterized by slightly limp corolla margins in all three genotypes. The middle stage of senescence was characterized by wilting of the entire corolla limb. Corollas at the late stage of senescence were completely wilted and the corolla margins were beginning to dry. Flowers for the last stage of senescence were harvested just prior to corolla abscission.
Phosphorus remobilization is decreased in etr1-1 corollas compared to MD
Phosphorus content was measured during the natural (i.e. age-related) senescence of unpollinated MD and etr1-1 corollas to investigate the regulation of P remobilization by ethylene. On the day of flower opening (early non-senescing stage) the total P (μg corolla-1) content of Z00-35-10 corollas was slightly less than that of MD and 44568 corollas. P levels remained relatively constant in non-senescing corollas and decreases were detected by the middle stage of senescence in all genotypes (Fig. 1B). The P content of naturally senescing corollas was similar in the middle and late stages of senescence. When compared to the P content of corollas at the early non-senescing stage, the P levels in MD corollas at the late senescing stage had been reduced by 74%. By contrast, the P level of etr1-1 corollas had only been reduced by 35% and 29% in Z00-35-10 and 44568 lines, respectively. The P differences observed between non-senescing and senescing (both middle and late stages) corollas of all three genotypes were significant at P <0.05. The differences in the total P content of MD, Z00-35-10 and 44568 corollas at the late senescing stage was also significant at P <0.05.
A phosphate transporter is identified from senescing corollas
Using RT-PCR and primers generated to a region that is highly conserved among phosphate transporter genes, a putative high-affinity phosphate transporter was cloned from senescing corolla cDNA. The original PCR fragment was 609 bp in length. The full-length cDNA of PhPT1 (PhPht1;1) was obtained by rapid amplification of cDNA ends (RACE) (SMART RACE kit, Clontech, Mountain View, CA). PhPT1 (GenBank Accession no. EF564180) contains a single open reading frame of 1605 nucleotides encoding a 58.6 kDa protein with 534 amino acid residues (ExPASy; Gasteiger et al., 2003). Comparisons of the nucleotide and deduced amino acid sequences of PhPT1 with sequences in GenBank revealed a high degree of similarity to the Pht1 family of high-affinity phosphate transporters in plants. Multiple sequence alignment with other phosphate transporters showed that PhPT1 contains the consensus sites for phosphorylation by protein kinase C and casein kinase II (see Supplementary Fig. S1 at JXB online). The PhPT1 sequence also contains 12 transmembrane domains and the Pht1 signature GGDYPLSATIxSE (Karandashov and Bucher, 2005) (see Supplementary Fig. S1 at JXB online).
Phylogenetic analysis demonstrated that PhPT1 is an orthologue of the Pht1;1 genes in tomato (LePT1) and other Solanaceous species (Fig. 2). PhPT1 shares 92%, 90%, and 83% amino acid identity with the high-affinity phosphate transporters from tobacco (NtPT1 or NtPht1;1; Chen et al., 2007), tomato (LePT1; Liu et al., 1998), and Arabidopsis (AtPT4; Mudge et al., 2002). The petunia phosphate transporter identified from senescing corollas (PhPT1) is nearly identical (99% nucleotide identity with only 1 nucleotide difference) to the partial sequence (GenBank Accession no. EU32760) recently published by Wegmuller et al. (2008). PhPT1 shares 80%, 82%, 58%, and 58% amino acid identity with the petunia phosphate transporters PhPT2 (partial sequence), PhPT3, PhPT4, and PhPT5, respectively (Wegmuller et al., 2008).
Fig. 2.
Phylogenetic tree of phosphate transporters that are members of the Pht1 family in Solanaceous species and Arabidopsis. The plants and genes (GenBank Accession no.) included are the following: eggplant, SmPT1 (EF091664), SmPT2 (EF091666), SmPT3 (EF091668), SmPT4 (EF091671), and SmPT5 (EF091674); pepper, CfPT1 (EF091663), CfPT2 (EF091665), CfPT3 (EF091667), CfPT4 (EF091670), and CfPT5 (EF091673); petunia, PhPT1 (EF564180), PhPT2 (EU532761), PhPT3 (EU532762), PhPT4 (EU532763), and PhPT5 (EU532764); potato, StPT1 (X98890), StPT2 (X98891), StPT3 (AJ318822), StPT4 (AY793559), and StPT5 (AY885654); tobacco, NtPT1 (AB020061), NtPT1.2 (AB042950), NtPT2 (AB042951), NtPT2.1 (AB042956), NtPT3 (EF091669), NtPT4 (EF091672), and NtPT5 (EF091675); tomato, LePT1 (AF022873), LePT2 (AF022874), LePT3 (AY804011), LePT4 (AY885652), and LePT5 (AY885653); and Arabidopsis, AtPht1 (AY070432), AtPht2 (At5g43370), AtPht3 (At5g43360), AtPht4 (AK226783), AtPht5 (AK117670), AtPht6 (At5g43340), AtPht7 (At3g54700), AtPht8 (At1g20860), and AtPht9 (At1g76430). The Neighbor–Joining tree was constructed by the MEGA 4.0 software with bootstrap values 0–100 (Tamura et al., 2007).
Increases in PhPT1 transcript abundance correspond with decreases in corolla P content
Real-time RT-PCR was used to quantify expression of PhPT1 during natural senescence in MD and etr1-1 corollas using flowers that were collected at the same time as those used for the nutrient analysis presented above. Gene expression was normalized with actin (PhACTIN) to give the relative expression of PhPT1. Transcript abundance of PhPT1 had increased slightly in Z00-35-10 corollas by the middle non-senescing stage, while transcript abundance increased in MD and 44568 at the late non-senescing stage (Fig. 1C). At the early senescing stage, PhPT1 expression was highest in Z00-35-10. Transcript abundance increased in all genotypes by the middle senescing stage. PhPT1 transcript abundance at the middle senescing stage was highest in MD corollas and represented an 85-fold increase from the early non-senescing stage. While PhPT1 levels were lower in both etr1-1 lines compared to MD, transcript levels were 3-fold higher in senescing Z00-35-10 compared to senescing 44568 corollas. The differences in relative transcript abundance at the middle senescing stage were significant for all genotypes at P <0.05. Gene expression of PhPT1 was not determined in late senescing corollas because the quality of the RNA obtained from this stage was not adequate for quantitative RT-PCR.
PhPT1 is a primary ethylene response gene
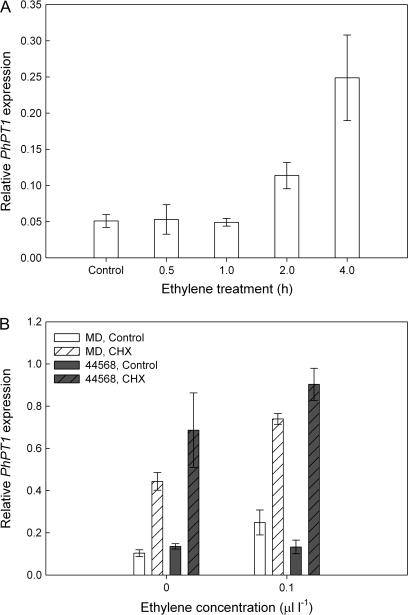
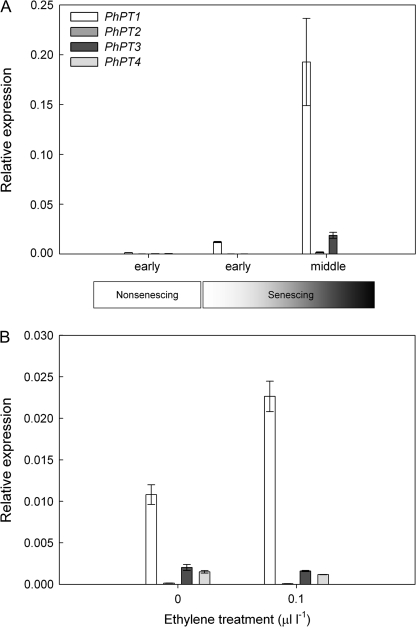
Detached MD flowers were treated with air (0 μl l-1) or 0.1 μl l-1 ethylene for 0.5, 1, 2, or 4 h, and mRNA levels for PhPT1 were determined in the corollas using quantitative RT-PCR. PhPT1 mRNA levels showed an early response to low concentrations of ethylene (Fig. 3A). Ethylene treatment induced PhPT1 transcripts 2-fold at 2 h and further transcript accumulation was detected at 4 h. Transcript levels were similar in flowers held in air for 0.5–4 h and only the 0.5 h air treatment (control) is shown in Fig. 3A. Treating flowers with higher doses of ethylene (1.0 or 10.0 μl l-1 for 4 h) did not result in further increases in transcript abundance (data not shown).
Fig. 3.
Ethylene-dependent and protein synthesis-independent regulation of PhPT1 expression in petunia corollas. (A) PhPT1 transcript levels (mean ±SD, n=3) in MD corollas following 0.5, 1, 2, and 4 h of treatment with 0.1 μl l-1 ethylene. Control flowers were held in air for 0.5 h. Relative mRNA abundance compared to PhACTIN was determined by quantitative RT-PCR. (B) PhPT1 transcript levels (mean ±SD, n=3) in MD and 44568 corollas following treatment with the protein synthesis inhibitor, cycloheximide (CHX). The cut peduncles of detached flowers were placed in a vase solution of dH2O or 50 μM CHX and exposed to 0 or 0.1 μl l-1 ethylene for 4 h.
The induction of primary response genes does not require de novo protein synthesis. To determine if ethylene-induced PhPT1 transcript accumulation was a primary response to ethylene, flowers were treated with the protein synthesis inhibitor cycloheximide (50 μM CHX). Treatment with 0.1 μl l-1 ethylene for 4 h increased transcript abundance in MD but not 44568 corollas (Fig. 3B). In the absence of ethylene, treatment with CHX increased the accumulation of PhPT1 transcripts in both MD and 44568 corollas. Treatment with ethylene and CHX increased transcript abundance in MD corollas 3-fold compared to ethylene treatment alone.
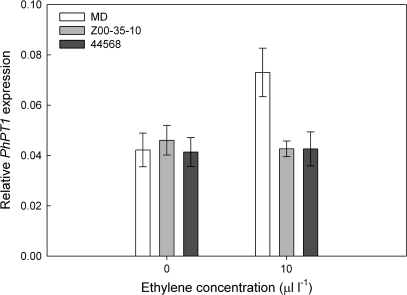
To determine if ethylene also regulated PhPT1 expression in vegetative tissues, 8-week-old petunias were treated with 10 μl l-1 ethylene for 4 h. Steady-state levels of PhPT1 mRNAs were elevated by ethylene treatment in MD but not etr1-1 leaves (Fig. 4). While this ethylene dosage was enough to induced PhPT1 expression, it was not sufficient to induce leaf senescence in either genotype (data not shown).
Fig. 4.
Ethylene-dependent regulation of PhPT1 expression in petunia leaves. Eight-week-old MD and etr1-1 (lines 44568 and Z00-35-10) petunia plants were treated with air or 10.0 μl l-1 ethylene for 4 h. Relative PhPT1 mRNA abundance (mean ±SD, n=3) compared to PhACTIN was determined by quantitative RT-PCR.
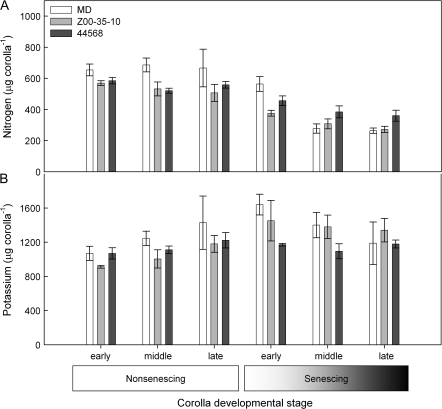
Nitrogen is remobilized during the senescence of MD and etr1-1 corollas
The nitrogen and potassium content of petunia corollas has also been shown to decline during senescence (Verlinden, 2003). To determine if N or K remobilization was also regulated by ethylene, the N and K content of MD and etr1-1 corollas was compared at the six stages of development and senescence previously described (Fig. 1A). Differential changes in nitrogen remobilization were observed between MD and etr1-1 corollas, but these differences were not as great as those observed with P (Figs 5A, 1B). On the day of flower opening (early non-senescing stage) the total N (μg corolla-1) content of MD corollas was higher than etr1-1 corollas. N levels remained relatively constant in non-senescing corollas. A decrease was first detected in Z00-35-10 and 44568 corollas at the early senescing stage, which was at 18 d and 22 d after flower opening, respectively. A decrease in corolla N content was detected at the middle and late senescing stages in all genotypes (significant at P <0.05). By the late senescing stage, N levels had been reduced by 60% in MD corollas, 52% in Z00-35-10 corollas and 38% in 44568 corollas. The total N content of senescing (late stage) corollas was significantly different between the etr1-1 lines Z00-35-10 and 44568 (P <0.05). By contrast, K levels increased steadily in all genotypes until the early senescing stage (Fig. 5B). At the late stage of senescence, K levels per corolla were higher than those measured from corollas on the day of flower opening in both MD and etr1-1 lines (P <0.05).
Fig. 5.
Changes in the nitrogen and potassium content of MD and etr1-1 corollas during the development and senescence of unpollinated flowers. (A) Corolla nitrogen content (mean ±SD, n=3) in MD and etr1-1 transgenic lines (Z00-35-10 and 44568). (B) Corolla potassium content (mean ±SD, n=3) in MD and etr1-1 transgenic lines (Z00-35-10 and 44568).
Petunia phosphate transporter genes are differentially regulated by ethylene and during senescence
Five genes encoding high-affinity phosphate transporters have recently been isolated from petunia (Wegmuller et al., 2008; this paper). To investigate senescence-induction and ethylene regulation in MD petunias, the expression of PhPT2, PhPT3, PhPT4, and PhPT5 was compared to that of PhPT1 in non-senescing and senescing corollas and in ethylene- and air-treated leaves and corollas. As was shown in Fig. 1C, PhPT1 was up-regulated during corolla senescence (Fig. 6A). PhPT2, PhPT3, and PhPT4 transcripts were barely detectable in non-senescing (day of flower opening) corollas. PhPT2 and PhPT4 transcript levels remained the same or decreased in senescing corollas. PhPT3 transcripts increased at the middle senescing stage (12 d after flower opening), but mRNA levels were more than 10-fold lower than those of PhPT1. PhPT1 was the only PhPT gene that was induced by ethylene in corollas (Fig. 6B). PhPT2, PhPT3, and PhPT4 were expressed at very low levels in untreated leaves, but transcript abundance was not up-regulated by ethylene treatment (data not shown). PhPT5 mRNAs were not detectable in corollas or leaves.
Fig. 6.
Expression of the Pht1 family of petunia phosphate transporters during corolla senescence and following ethylene treatment. Quantitative RT-PCR was conducted using primers for PhPT1, PhPT2, PhPT3, PhPT4, and PhPT5. Expression of the individual PhPT genes was determined relative to PhACTIN. PhPT5 transcripts were not detectable in corollas. (A) Expression of PhPTs (mean ±SD, n=3) in corollas on the day of flower opening (non-senescing) and at the early and middle stages of senescence. (B) Expression of PhPTs in corollas following treatment with 0 or 0.1 μl l-1 ethylene for 4 h.
Discussion
A genetically controlled senescence programme allows plants to salvage valuable nutrients from dying organs. There is significant evidence that this is the central role of senescence, as many senescence-enhanced genes in both leaves and petals encode for catabolic enzymes involved in the breakdown of macromolecules and cell organelles (Jones, 2004; van Doorn and Woltering, 2008). Since nutrient remobilization requires energy, the specific nutrients and the levels that can be remobilized from senescing organs must result in a net advantage to the growth and development of the plant. In many plants, ethylene serves as the hormone signal that initiates the senescence programme in leaves and flowers. This ethylene may result from the normal increases in endogenous ethylene synthesis that accompanies growth and development or it may be prematurely induced as a result of pollination, abiotic or biotic stresses. While many senescence- enhanced genes encoding catabolic enzymes have been found to be regulated by ethylene, comparatively little is known about ethylene's role in nutrient remobilization during the later stages of senescence.
The nitrogen and phosphorus content of wild-type ‘Mitchell Diploid’ corollas declined during the later stages of flower development, suggesting that remobilization of these nutrients occurs during petal senescence. From flower opening to late senescence, the corolla P content declined by 74% and the N content declined by 60%. Similar changes in the mineral nutrient content of senescing MD corollas have previously been reported (Chapin and Jones, 2007; Jones, 2008; Verlinden, 2003). Our current studies using transgenic petunias with reduced sensitivity to ethylene (35S::etr1-1; lines Z00-35-10 and 44568) were aimed at identifying ethylene's role in nutrient remobilization. In contrast to MD corollas, P levels in etr1-1 lines were reduced by only 35% and 29% in the Z00-35-10 and 44568 lines, respectively. While 92 μg P per corolla was remobilized from MD corollas, an average of only 36 μg P per corolla was remobilized from etr1-1 corollas. The differences in N remobilization between MD and etr1-1 corollas were not as great as those of P. Nitrogen levels in MD corollas decreased by 60% compared to an average decrease of 45% in etr1-1 lines. These experiments confirm that ethylene perception enhances nutrient transport during the remobilization phase of senescence and suggest that a central role of petal senescence is P recycling.
Endonucleases, working in concert with phosphatases and phosphodiesterases, release phosphate from DNA and RNA for remobilization during senescence (Perez-Amador et al., 2000). Induction of nuclease and RNase activity, decreases in nucleic acid content, and DNA fragmentation accompany petal and leaf senescence in many species including petunia (Taylor and Green, 1991; Taylor et al., 1993; Lers et al., 1998, 2001; Panavas et al., 2000; Perez-Amador et al., 2000; Xu and Hanson, 2000; Langston et al., 2005). Some of these senescence-enhanced nucleases are also induced during phosphate starvation (Taylor et al., 1993; Kock et al., 1995; Lers et al., 1998; Liang et al., 2002). The tomato LX S-like RNase is induced by phosphate starvation (Kock et al., 1995), during leaf senescence and following ethylene treatment of young leaves (Lers et al., 1998). Recently, the antisense suppression of LX RNase in transgenic tomatoes was shown to delay leaf senescence, supporting an important functional role for nucleases and Pi salvage during the senescence programme (Lers et al., 2006).
To investigate Pi remobilization during corolla senescence further and to determine the role of ethylene signalling in this process, a putative phosphate transporter was cloned from senescing petunia corollas (PhPT1). PhPT1 shows a high degree of similarity to known high-affinity phosphate transporters in plants, and appears to be an orthologue of tobacco NtPT1 (NtPht1;1) and tomato LePT1 (LePht1;1). Both LePT1 and NtPT1 have been shown to encode functional phosphate transporters by yeast complementation assays (Daram et al., 1998; Baek et al., 2001).
High-affinity plant Pi transporters belong to the Phosphate Transporter1 (Pht1) family of Pi/H+ symporters (Rausch and Buchner, 2002). The central role of high-affinity transporters is Pi acquisition during Pi starvation. In support of this role, most of the plant Pht1 transporters characterized to date are expressed in roots and are induced by Pi starvation (Mudge et al., 2002; Raghothama and Karthikeyan, 2005; Chen et al., 2007). While phosphate transporters are transcriptionally regulated by Pi availability and uptake, phosphorus transport and utilization within the plant also influences the P status of the plant and results in the transcriptional regulation of members of the Pht1 family (Muchhal and Raghothama, 1999; Smith, 2002). Expression of some of these high-affinity phosphate transporters has been reported in leaves and flowers, supporting a role in Pi translocation within the plant (Leggewie et al., 1997; Liu et al., 1998; Karthikeyan et al., 2002; Mudge et al., 2002).
Among the petunia high-affinity phosphate transporters (PhPT1–PhPT5; this paper and Wegmuller et al., 2008), PhPT1 had the highest steady-state mRNA levels in corollas and was up-regulated during petal senescence. Increased PhPT1 transcript abundance corresponded with decreased P content in the petals. Wegmuller and colleagues (2008) recently reported that PhPT4 shows mycorrhiza-specific expression while PhPT3 and PhPT5 are expressed at low levels in roots and are mycorrihiza-inducible. Low levels of PhPT1 and PhPT2 are also detectable in roots but expression of these genes is not altered by mycorrhizal colonization. The senescence and ethylene-inducible expression of PhPT1 suggests that it may play a role in Pi remobilization during senescence, while the other phosphate transporters (PhPT2–PhPT5) may have a primary role in Pi uptake.
While there is increasing evidence that individual members of the Pht1 transporter family play different roles in the uptake and/ or translocation of Pi, most phosphate transporters have been identified from roots and expression of only a few of them has been investigated during natural or stress-induced senescence. NtPT1/2 mRNAs were reported to be higher in mature leaves than immature leaves or stems, suggesting that the tobacco orthologue of PhPT1 may play a role in Pi remobilization during leaf senescence (Kai et al., 2002). Unfortunately, the expression of Pht1;1 in senescing leaves has not been investigated in tomato, potato, pepper or eggplant. Five of the nine members of the Arabidopsis Pht1 family of phosphate transporters are expressed in other tissues in addition to the roots. Arabidopsis plants transformed with AtPht1;5 promoter:GUS constructs show no GUS activity in leaves until they start to senesce, at which time activity is localized to the vascular tissue (phloem) (Mudge et al., 2002). GUS activity is also detected in young floral buds, suggesting that AtPht1;5 may play a role in remobilization of Pi from older leaves to newly developing flowers (Mudge et al., 2002). Recent transcript profiling experiments in Arabidopsis have confirmed that AtPht1;5 is up regulated during the natural senescence of leaves (van der Graaff et al., 2006) and remobilization of P from senescing Arabidopsis leaves has been demonstrated (Himelblau and Amasino, 2001). To the best of our knowledge, expression of Pht1 genes has not been reported in senescing petals.
There are reports that suggest ethylene plays a role in the phosphate starvation response by altering root morphology, but there has been little, if any, evidence that ethylene regulates the expression of phosphate transporter genes (Schmidt, 2001; Raghothama and Karthikeyan, 2005). While cytokinins and auxin repress expression of Arabidopsis AtPT1 (AtPht1;1), application of the ethylene precursor, 1-aminocyclopropane-1-carboxylic acid, has no affect on expression of AtPT1 in seedlings under Pi-sufficient or Pi-deficient conditions (Karthikeyan et al., 2002). By contrast, expression of the petunia phosphate transporter, PhPT1, was up-regulated in corollas following treatment with 0.1 μl l-1 ethylene for only 2 h. Senescence-related increases in PhPT1 transcript abundance were much lower in etr1-1 petunias compared to MD, and the remobilization of Pi from senescing etr1-1 corollas was also reduced. The level of PhPT1 expression was higher in etr1-1 Z00-35-10 than 44568 during petal senescence, and a greater reduction in P levels was also measured in Z00-35-10 corollas (35% reduction in Z00-35-10 compared to a 29% reduction in 44568; significant at P <0.05). Z00-35-10 and 44568 have moderate and strong reductions in ethylene sensitivity, respectively (Shibuya et al., 2004; ML Jones, unpublished data). These differences in ethylene responsiveness could explain the differences in PhPT1 expression and Pi remobilization observed between the two etr1-1 lines and further support a role for ethylene signalling in Pi remobilization during petal senescence. While the changes in P levels observed in the etr1-1 lines may be the result of residual ethylene perception, it is also possible that this basal level of nutrient remobilization is ethylene independent.
The rapid induction of PhPT1 by low levels of ethylene suggests that it is an early or primary response gene. Primary response genes can be induced in the absence of de novo protein synthesis. Treatment with the protein synthesis inhibitor, cycloheximide (CHX), did not inhibit the ethylene-induced expression of PhPT1, confirming that PhPT1 is an ethylene primary response gene. While many primary response genes encode transcription factors, there are an increasing number of examples of primary response genes that function as effectors rather than mediators of hormone signalling (Sauter et al., 2005).
PhPT1 transcripts were also superaccumulated following CHX treatment in the absence of ethylene. CHX hyperinduction is commonly observed with primary response genes and may be the result of either transcriptional activation or increased mRNA stability (Suzuki et al., 1998). It has been proposed that CHX prevents the synthesis of short-lived transcriptional repressor proteins and that primary response genes are then activated following the loss of repressor function. Messenger RNA stability may also be enhanced if the synthesis of a labile nuclease involved in mRNA degradation is prevented by CHX. Ethylene perception was not required for the superinduction of PhPT1 by CHX, as similar transcript hyperaccumulation was detected in etr1-1 and MD corollas following CHX treatment. These results suggest that PhPT1 gene expression may be controlled by a transcriptional repressor.
In petunia, the ethylene signal that initiates senescence results in the transcriptional activation of the high-affinity phosphate transporter, PhPT1, which functions in the remobilization of Pi during the later stages of senescence. Since P can be the most growth-limiting nutrient, this allows the plant to use nucleic acids as P storage molecules and to respond rapidly to senescence signals by remobilizing Pi from unneeded tissues like corollas or older leaves. Evidence so far supports the involvement of both hormone-dependent and -independent signalling pathways in the Pi responses that lead to increased Pi uptake and reallocation within the plant. Hormone signals during natural senescence and remobilization of Pi from plant organs may involve signalling pathways that are distinct from those functioning during the Pi starvation responses in the shoot and root.
Supplementary data
The following supplementary data for this article are available at JXB online.
Supplementary Table S1. Primers used for the quantitative RT-PCR of the PhPT genes and the PhACTIN control.
Supplementary Fig. S1. Alignment of Pht1 phosphate transporters.
Supplementary Material
Acknowledgments
This research was funded by the USDA Floriculture and Nursery Research Initiative and the Fred C Gloeckner Foundation. Salaries and research support were provided, in part, by State and Federal funds appropriated to the Ohio Agricultural Research and Development Center, The Ohio State University. Journal Article Number HCS 07-09. We would like to thank Dr Tea Meulia (Molecular and Cellular Imaging Center, OARDC) for use of the Bio-Rad iQ5 thermocycler; Dr David Clark (University of Florida) for the etr1-1 petunia seeds; Dr Shuangyi Bai for his efforts in the initial cloning of PhPT1; and Eileen Ramsay for her assistance in the greenhouse and laboratory. We would also like to acknowledge Drs Kashchandra Ragothama (Purdue University) and Stephen Mudge (CSIRO, Australia) for providing us with tomato and Arabidopsis Pht1 sequences.
References
- Baek SH, Chung IM, Yun SJ. Molecular cloning and characterization of a tobacco leaf cDNA encoding a phosphate transporter. Molecules and Cells. 2001;11:1–6. [PubMed] [Google Scholar]
- Borochov A, Woodson WR. Physiology and biochemistry of flower petal senescence. Horticultural Reviews. 1989;11:15–43. [Google Scholar]
- Buchanan-Wollaston V, Ainsworth C. Leaf senescence in Brassica napus: cloning of senescence-related genes by subtractive hybridization. Plant Molecular Biology. 1997;33:821–834. doi: 10.1023/a:1005774212410. [DOI] [PubMed] [Google Scholar]
- Buchanan-Wollaston V, Earl S, Harrison E, Mathas E, Navabpour S, Page T, Pink D. The molecular analysis of leaf senescence. Plant Biotechnology Journal. 2003;1:3–22. doi: 10.1046/j.1467-7652.2003.00004.x. [DOI] [PubMed] [Google Scholar]
- Bucher M. Functional biology of plant phosphate uptake at root and mycorrhiza interfaces. New Phytologist. 2007;173:11–26. doi: 10.1111/j.1469-8137.2006.01935.x. [DOI] [PubMed] [Google Scholar]
- Chapin L, Jones ML. Nutrient remobilization during pollination-induced corolla senescence in petunia. Acta Horticulturae. 2007;755:181–190. [Google Scholar]
- Chen A, Hu J, Sun S, Xu G. Conservation and divergence of both phosphate- and mycorrhiza-regulated physiological responses and expression patterns of phosphate transporters in Solanaceous species. New Phytologist. 2007;173:817–831. doi: 10.1111/j.1469-8137.2006.01962.x. [DOI] [PubMed] [Google Scholar]
- Claros MG, von Heijne G. TopPred II: an improved software for membrane protein structure predictions. Computer Applications in the Biosciences. 1994;10:685–686. doi: 10.1093/bioinformatics/10.6.685. [DOI] [PubMed] [Google Scholar]
- Daram P, Brunner S, Persson BL, Amrhein N, Bucher M. Functional analysis and cell-specific expression of a phosphate transporter from tomato. Planta. 1998;206:225–233. doi: 10.1007/s004250050394. [DOI] [PubMed] [Google Scholar]
- Eason JR, Johnston JW, de Vre L, Sinclair BK, King GA. Amino acid metabolism in senescing Sandersonia aurantiaca flowers: cloning and characterization of asparagine synthetase and glutamine synthetase cDNAs. Australian Journal of Plant Physiology. 2000;27:389–396. [Google Scholar]
- Gasteiger E, Gattiker A, Hoogland C, Appel RD, Bairoch A. ExPASy: the proteomics server for in-depth protein knowledge and analysis. Nucleic Acids Research. 2003;31:3784–3788. doi: 10.1093/nar/gkg563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gubrium EK, Clevenger DJ, Clark DG, Barrett JE, Nell TA. Reproduction and horticultural performance of transgenic ethylene-insensitive petunias. Journal of the American Society for Horticultural Science. 2000;125:277–281. [Google Scholar]
- Himelblau E, Amasino RM. Nutrients remobilized from leaves of Arabidopsis thaliana during leaf senescence. Journal of Plant Physiology. 2001;158:1317–1323. [Google Scholar]
- Isaac RA, Johnson WA. Elemental analysis of plant tissue by plasma emission spectroscopy: collaborative study. Journal of the Association of Official Analytical Chemists. 1985;68:499–505. [Google Scholar]
- Jones ML. Changes in gene expression during senescence. In: Noodén L, editor. Plant cell death processes. San Diego, CA: Elsevier Science; 2004. pp. 51–72. [Google Scholar]
- Jones ML. Ethylene signalling is required for pollination-accelerated corolla senescence in petunias. Plant Science. 2008;175:190–196. [Google Scholar]
- Jones ML, Chaffin GS, Eason JR, Clark DG. Ethylene sensitivity regulates proteolytic activity and cysteine protease gene expression in petunia corollas. Journal of Experimental Botany. 2005;56:2733–2744. doi: 10.1093/jxb/eri266. [DOI] [PubMed] [Google Scholar]
- Kai M, Takazumi K, Adachi H, Wasaki J, Shinano T, Osaki M. Cloning and characterization of four phosphate transporter cDNAs in tobacco. Plant Science. 2002;163:837–846. [Google Scholar]
- Kamachi K, Yamaya T, Hayakawa T, Mae T, Ojima K. Changes in cytosolic glutamine synthetase polypeptide and its mRNA in a leaf blade of rice plants during natural senescence. Plant Physiology. 1992;98:1323–1329. doi: 10.1104/pp.98.4.1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karandashov V, Bucher M. Symbiotic phosphate transport in arbuscular mycorrhizas. Trends in Plant Science. 2005;10:22–29. doi: 10.1016/j.tplants.2004.12.003. [DOI] [PubMed] [Google Scholar]
- Karthikeyan AS, Varadarajan DK, Mukatira UT, D'Urzo MP, Damsz B, Raghothama KG. Regulated expression of Arabidopsis phosphate transporters. Plant Physiology. 2002;130:221–223. doi: 10.1104/pp.020007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kock M, Loffier A, Abel S, Glund K. Structural and regulatory properties of a family of phosphate starvation induced ribonucleases from tomato. Plant Molecular Biology. 1995;27:477–485. doi: 10.1007/BF00019315. [DOI] [PubMed] [Google Scholar]
- Langston BL, Bai S, Jones ML. Increases in DNA fragmentation and induction of a senescence-specific nuclease are delayed during the senescence of ethylene-insensitive (etr1-1) transgenic petunias. Journal of Experimental Botany. 2005;56:15–23. doi: 10.1093/jxb/eri002. [DOI] [PubMed] [Google Scholar]
- Larkin MA, Blackshields G, Brown NP, et al. Clustal W and Clustal X version 2. Bioinformatics. 2007;23:2947–2948. doi: 10.1093/bioinformatics/btm404. [DOI] [PubMed] [Google Scholar]
- Leggewie G, Willmitzer L, Riesmeier JW. Two cDNAs from potato are able to complement a phosphate uptake-deficient yeast mutant: identification of phosphate transporters from higher plants. The Plant Cell. 1997;9:381–392. doi: 10.1105/tpc.9.3.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lers A, Khalchitski A, Lomaniec E, Burd S, Green PJ. Senescence-induced RNases in tomato. Plant Molecular Biology. 1998;36:439–449. doi: 10.1023/a:1005993024161. [DOI] [PubMed] [Google Scholar]
- Lers A, Lomaniec E, Burd S, Khalchitski A. The characterization of LeNUC1, a nuclease associated with leaf senescence in tomato. Physiologia Plantarum. 2001;112:176–182. doi: 10.1034/j.1399-3054.2001.1120205.x. [DOI] [PubMed] [Google Scholar]
- Lers A, Sonego L, Green PJ, Burd S. Suppression of LX ribonuclease in tomato results in a delay in leaf senescence and abscission. Plant Physiology. 2006;142:710–721. doi: 10.1104/pp.106.080135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang L, Lai Z, Ma W, Zhang Y, Xue Y. AhSL28, a senescence-and phosphate starvation-induced S-like RNase gene in Antirrhinum. Biochimica et Biophysica Acta. 2002;1579:64–71. doi: 10.1016/s0167-4781(02)00507-9. [DOI] [PubMed] [Google Scholar]
- Liu C, Muchhal US, Uthappa M, Kononowicz AK, Raghothama KG. Tomato phosphate transporter genes are differentially regulated in plant tissues by phosphorus. Plant Physiology. 1998;116:91–99. doi: 10.1104/pp.116.1.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muchhal US, Raghothama KG. Transcriptional regulation of plant phosphate transporters. Proceedings of the National Academy of Sciences. 1999;96:5868–5872. doi: 10.1073/pnas.96.10.5868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mudge SR, Rae AL, Diatloff E, Smith FW. Expression analysis suggests novel roles for members of the Pht1 family of phosphate transporters in Arabidopsis. The Plant Journal. 2002;31:341–353. doi: 10.1046/j.1365-313x.2002.01356.x. [DOI] [PubMed] [Google Scholar]
- Panavas T, LeVangie R, Mistler J, Reid PD, Rubinstein B. Activities of nucleases in senescing daylily petals. Plant Physiology and Biochemistry. 2000;38:837–843. [Google Scholar]
- Perez-Amador MA, Abler ML, De Rocher EJ, Thompson DM, van Hoof A, LeBrasseur ND, Lers A, Green PJ. Identification of BFN1, a bifunctional nuclease induced during leaf and stem senescence in Arabidopsis. Plant Physiology. 2000;122:169–179. doi: 10.1104/pp.122.1.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rae AL, Cybinski DH, Jarmey JM, Smith FW. Characterization of two phosphate transporters from barley; evidence for diverse function and kinetic properties among members of the Pht1 family. Plant Molecular Biology. 2003;53:27–36. doi: 10.1023/B:PLAN.0000009259.75314.15. [DOI] [PubMed] [Google Scholar]
- Raghothama KG, Karthikeyan AS. Phosphate acquisition. Plant and Soil. 2005;274:37–49. [Google Scholar]
- Rausch C, Bucher M. Molecular mechanisms of phosphate transport in plants. Planta. 2002;216:23–27. doi: 10.1007/s00425-002-0921-3. [DOI] [PubMed] [Google Scholar]
- Sauter M, Lorbiecke R, OuYang B, Pochapsky TC, Rzewuski G. The immediate-early ethylene response gene OsARD1 encodes an acireductone dioxygenase involved in recycling of the ethylene precursor S-adenosylmethionine. The Plant Journal. 2005;44:718–729. doi: 10.1111/j.1365-313X.2005.02564.x. [DOI] [PubMed] [Google Scholar]
- Schmidt W. From faith to fate: ethylene signaling in morphogenic responses to P and Fe deficiency. Journal of Plant Nutrition and Soil Science. 2001;164:147–154. [Google Scholar]
- Shibuya K, Barry KG, Ciardi JA, Loucas HM, Underwood BA, Nourizadeh S, Ecker JR, Klee HJ, Clark DG. The central role of PhEIN2 in ethylene responses throughout plant development in petunia. Plant Physiology. 2004;136:2900–2912. doi: 10.1104/pp.104.046979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith FW. The phosphate uptake mechanism. Plant and Soil. 2002;245:105–114. [Google Scholar]
- Stead AD, van Doorn WG, Jones ML, Wagstaff C. Flower senescence: fundamental and applied aspects. In: Ainsworth C, editor. Flowering and its manipulation. Annual Plant Reviews. Vol. 20. Oxford: Blackwell Publishing; 2006. pp. 261–296. [Google Scholar]
- Suzuki K, Suzuki N, Ohme-Takagi M, Shinshi H. Immediate early induction of mRNAs for ethylene-responsive transcription factors in tobacco leaf strips after cutting. The Plant Journal. 1998;15:657–665. doi: 10.1046/j.1365-313x.1998.00243.x. [DOI] [PubMed] [Google Scholar]
- Sweeney RA. Generic combustion method for determination of crude protein in feeds: collaborative study. Journal of the Association of Official Analytical Chemists. 1989;72:770–774. [PubMed] [Google Scholar]
- Tamura K, Dudley J, Nei M, Kumar S. MEGA4: molecular evolutionary genetics analysis (MEGA) software version 4.0. Molecular Biology and Evolution. 2007;24:1596–1599. doi: 10.1093/molbev/msm092. [DOI] [PubMed] [Google Scholar]
- Taylor CB, Bariola PA, del Cardayre SB, Raines RT, Green PJ. RNS2: A senescence associated RNase of Arabidopsis that diverged from the S-RNases before speciation. Proceedings of the National Academy of Sciences, USA. 1993;90:5118–5122. doi: 10.1073/pnas.90.11.5118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor CB, Green PJ. Genes with homology to fungal and S-gene RNases are expressed in Arabidopsis thaliana. Plant Physiology. 1991;96:980–984. doi: 10.1104/pp.96.3.980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Graaff E, Schwacke R, Schneider A, Desimone M, Flugge U-I, Kunze R. Transcription analysis of Arabidopsis membrane transporters and hormone pathways during developmental and induced leaf senescence. Plant Physiology. 2006;141:776–792. doi: 10.1104/pp.106.079293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Doorn WG, Woltering EJ. Physiology and molecular biology of petal senescence. Journal of Experimental Botany. 2008;59:453–480. doi: 10.1093/jxb/erm356. [DOI] [PubMed] [Google Scholar]
- Verlinden S. Changes in mineral nutrient concentrations in petunia corollas during development and senescence. HortScience. 2003;38:71–74. [Google Scholar]
- Wegmuller S, Svistoonoff S, Reinhardt D, Stuurman J, Amrhein N, Bucher B. A transgenic dTph1 insertional mutagenesis system for forward genetics in mycorrhizal phosphate transport of Petunia. The Plant Journal. 2008;54:1115–1127. doi: 10.1111/j.1365-313X.2008.03474.x. [DOI] [PubMed] [Google Scholar]
- Wilkinson JQ, Lanahan MB, Clark DG, Bleecker AB, Chang C, Meyerowitz EM, Klee HJ. A dominant mutant receptor from Arabidopsis confers ethylene insensitivity in heterologous plants. Nature Biotechnology. 1997;15:444–447. doi: 10.1038/nbt0597-444. [DOI] [PubMed] [Google Scholar]
- Woltering EJ, van Doorn WG. Role of ethylene in the senescence of petals: morphological and taxonomical relationships. Journal of Experimental Botany. 1988;39:1605–1616. [Google Scholar]
- Xu Y, Hanson MR. Programmed cell death during pollination-induced petal senescence in petunia. Plant Physiology. 2000;122:1323–1333. doi: 10.1104/pp.122.4.1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.