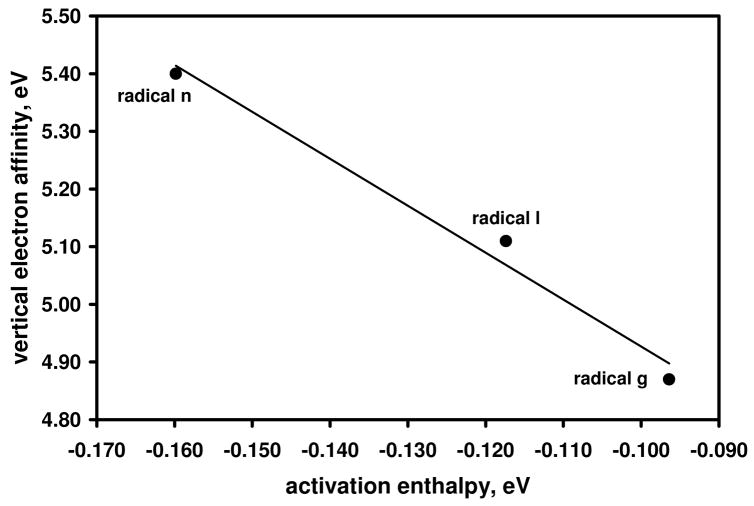
Figure 6.
Calculated ((U)B3LYP/6-31+G(d)) vertical electron affinities (eV) versus calculated (UMPW1K/6-31+G(d,p)) activation enthalpies (eV) for aryl radicals g, l and n. The activation enthalpies are the differences in enthalpies between the separated reactants and the transition state. The data are fit to a linear trend line (R2 = 0.98).