Abstract
Primary cilia are found on many epithelial cell types, including renal tubular epithelial cells, in which they are felt to participate in flow sensing and have been linked to the pathogenesis of cystic renal disorders such as autosomal dominant polycystic kidney disease. We previously localized the exocyst, an eight-protein complex involved in membrane trafficking, to the primary cilium of Madin-Darby canine kidney cells and showed that it was involved in cystogenesis. Here, using short hairpin RNA (shRNA) to knockdown exocyst expression and stable transfection to induce exocyst overexpression, we show that the exocyst protein Sec10 regulates primary ciliogenesis. Using immunofluorescence, scanning, and transmission electron microscopy, primary cilia containing only basal bodies are seen in the Sec10 knockdown cells, and increased ciliogenesis is seen in Sec10-overexpressing cells. These phenotypes do not seem to be because of gross changes in cell polarity, as apical, basolateral, and tight junction proteins remain properly localized. Sec10 knockdown prevents normal cyst morphogenesis when the cells are grown in a collagen matrix, whereas Sec10 overexpression results in increased cystogenesis. Transfection with human Sec10 resistant to the canine shRNA rescues the phenotype, demonstrating specificity. Finally, Par3 was recently shown to regulate primary cilia biogenesis. Par3 and the exocyst colocalized by immunofluorescence and coimmunoprecipitation, consistent with a role for the exocyst in targeting and docking vesicles carrying proteins necessary for primary ciliogenesis.
INTRODUCTION
Cilia are thin rod-like organelles, found on the surface of many eukaryotic cells, which extend outward from the basal body, a cellular organelle related to the centriole. Cilia are classified as primary (nonmotile) or motile and contain a central axoneme composed of microtubules. In kidney cells, the primary cilium projects from the basal body, is nonmotile, and exhibits an axoneme microtubular pattern of 9 + 0. This is in contrast to motile cilia that exhibit a typical 9 + 2 axoneme microtubular pattern of organization. In epithelia containing numerous motile cilia, the cilia have been observed to have a propulsive function (Fawcett and Porter, 1954), whereas primary cilia are thought to have a mechanosensory function, with calcium acting as an intracellular second messenger (Smyth et al., 2003). In the mammalian kidney, primary cilia have been observed on cells in the parietal layer of Bowman's capsule, the proximal tubule, the distal tubule, and in the principal, but not intercalated, cells of the collecting duct (Webber and Lee, 1975).
Structurally, cilia are covered by a membrane that is continuous with the apical plasma membrane, although it remains unclear as to whether the ciliary membrane is identical to the apical membrane in composition. Importantly, the primary cilium of the kidney has been implicated in the pathogenesis of polycystic kidney disease (PKD). Several gene products, which when mutated result in the development of PKD, including polycystin-1 and polycystin-2, have been localized to and are crucial for the function of renal primary cilia (for review, see Smyth et al., 2003). It was recently shown that the postsynaptic density 95/disc-large/zona occludens (PDZ) protein Par3 is necessary for the biogenesis of primary renal cilia (Fan et al., 2004; Sfakianos et al., 2007).
The exocyst is a highly conserved complex composed of eight proteins: Sec3, Sec5, Sec6, Sec8, Sec10, Sec15, Exo70, and Exo84. The exocyst was originally identified in budding yeast Saccharomyces cerevisiae, where it was shown to be essential for exocytosis. The eight proteins in the exocyst complex, six “Sec” proteins, so named because mutations inhibit secretion (Novick et al., 1980), and two additional subunits, Exo70 and Exo84, were purified and shown to physically interact (Terbush et al., 1996). Mammalian homologues of all eight yeast exocyst proteins have been identified previously (Hsu et al., 1996). All of the exocyst components are hydrophilic proteins that form a 17S complex peripherally associated with the plasma membrane (Grindstaff et al., 1998).
In yeast, mutants of individual exocyst members accumulate secretory vesicles in cells, presumably because vesicles are not able to dock or fuse with the plasma membrane. The exocyst proteins are localized to regions of active cell surface expansion: the bud tip at the beginning of the cell cycle and the mother–daughter cell connection during cytokinesis. The exocyst is therefore thought, among other things, to be involved in tethering vesicles to their precise sites of fusion (Terbush et al., 1996; Finger and Novick, 1998; Guo et al., 1999).
We previously showed in Madin-Darby canine kidney (MDCK) cells that the exocyst complex was centrally involved in cyst formation. Specifically, we overexpressed the Sec10 component of the exocyst complex and found that cyst formation occurred more efficiently and rapidly than in untransfected cells (Lipschutz et al., 2000). We also showed that the exocyst complex localized to the primary cilium (Rogers et al., 2004), which was later confirmed by others (Liu et al., 2007). Importantly, in primary cultures of human autosomal dominant PKD (ADPKD) cells, the exocyst was depleted from the ADPKD cell membranes and seemed diffusely dispersed throughout the cytoplasm (Charron et al., 2000).
Given the localization of the exocyst to primary cilia, the involvement of the exocyst and primary cilia in cystogenesis, and the mislocalization of the exocyst in ADPKD, we hypothesized that the exocyst was centrally involved in cilia biogenesis. Here, we show that the exocyst component Sec10 regulates ciliogenesis and cystogenesis, most likely through interactions with Par3.
MATERIALS AND METHODS
Cell Culture
The parent cells for all the experiments were low passage type II MDCK cells that were obtained from Dr. K. Mostov (UCSF, San Francisco, CA) and used between passages 3 and 10. These cells were originally cloned by Dr. D. Louvard (European Molecular Biology Laboratory, Heidelberg, Germany) and came to Dr. Mostov via Dr. K. Matlin (University of Chicago, Chicago, IL). MDCK type II cells overexpressing hSec10 (Lipschutz et al., 2000), T23 MDCK cells (from K. Mostov) stably infected with exocyst shRNA, and control MDCK cells were cultured in modified Eagle's minimal essential medium containing Earl's balanced salt solution and glutamine supplemented with 5% fetal calf serum, 100 U/ml penicillin, and 100 μg/ml streptomycin. MDCK cells were seeded at confluence on 24-mm Transwell filter units coated with collagen (Corning Life Sciences, Lowell, MA). ARPE-19 cells (American Type Culture Collection, Manassas, VA) were cultured on Transwell filters in DMEM:F-12 medium supplemented with 10% fetal calf serum. Pore size on all filters was 0.4 μm. Cell monolayers were used for experiments after 7–14 d of culture with daily changes in medium.
MDCK cells overexpressing hSec10, exocyst shRNA-expressing cell lines, and control MDCK cells were plated as single cells in a three-dimensional (3D) type I collagen matrix as previously described previously (Lipschutz et al., 2000).
Short Hairpin RNA (shRNA) Oligonucleotides (Oligos)
Three shRNA oligos were created for canine Sec8, Sec10, and Exo70 by using the Elledge Lab pPRIME system, which they kindly sent us (Stegmeier et al., 2005). The shRNA sequences were designed by pasting the Sec10 canine mRNA sequences into the program found at the RNAi Central website (http://katahdin.cshl.org:9331/homepage/siRNA/RNAi.cgi?type=siRNA). The shRNA sequences were cloned into the p199 cloning vector and then into a lentiviral delivery system for infection into MDCK and ARPE-19 cells. The p199 vector encodes green fluorescent protein (GFP), which allowed us to identify and separate the infected cells by using fluorescence-activated cell sorting (FACS Vantage SE; BD Biosciences, San Jose, CA). The shRNA sequences are as follows: Exo70 shRNA1, TGCTGTTGACAGTGAGCGAACAGGCAGGCTGGAAGAGTACTAGTGAAGCCACAGATGTAGTACTCTTCCAGCCTGCCTGTGTGCCTACTGCCTCGGA; Exo70 shRNA2, TGCTGTTGACAGTGAGCGATCCCGCTGGCTGGTGGAATACTAGTGAAGCCACAGATGTAGTATTCCACCAGCCAGCGGGAGTGCCTACTGCCTCGGA; Exo70 shRNA3, TGCTGTTGACAGTGAGCGACCAGCTAGACCGCTCCATCAATAGTGAAGCCACAGATGTATTGATGGAGCGGTCTAGCTGGCTGCCTACTGCCTCGGA; Sec8 shRNA1, TGCTGTTGACAGTGAGCGATGTGACCGTGACCTGGATGAGTAGTGAAGCCACAGATGTACTCATCCAGGTCACGGTCACACTGCCTACTGCCTCGGA; Sec8 shRNA2, TGCTGTTGACAGTGAGCGAATAGAGGAACTGCACCGACATTAGTGAAGCCACAGATGTAATGTCGGTGCAGTTCCTCTATGTGCCTACTGCCTCGGA; Sec8 shRNA3, TGCTGTTGACAGTGAGCGAACAGTGAAGGCGATCAAAGAGTAGTGAAGCCACAGATGTACTCTTTGATCGCCTTCACTGTCTGCCTACTGCCTCGGA; Sec10 shRNA1, TGCTGTTGACAGTGAGCGAAGCAACAGTGTCAGAAAGAAGTAGTGAAGCCACAGATGTACTTCTTTCTGACACTGTTGCTCTGCCTAC TGCCTCGGA; Sec10 shRNA2, TGCTGTTGACAGTGAGCGAGCTCAGAAATTGATGAAATACTAGTGAAGCCACAGATGTAGTATTTCATCAATTTCTGAGCCTGCCTACTGCCTCGGA; and Sec10 shRNA3, TGCTGTTGACAGTGAGCGCTATGAGCATCTTCAACAATACTAGTGAAGCCACAGATGTAGTATTGTTGAAGATGCTCATAATGCCTACTGCCTCGGA.
Immunofluorescence Confocal Microscopy
Cells were grown on Transwell filters and fixed with 4% paraformaldehyde for 20 min on ice, permeabilized for 15 min at 37°C with 0.025% saponin in phosphate-buffered saline containing 0.7% fish skin gelatin (PFS buffer), and incubated with primary antibodies overnight at 4°C and secondary antibodies 1 h at 37°C.
Cysts grown in collagen gel were fixed with 4% paraformaldehyde for 30 min at 4°C after digesting in collagenase (100 U/ml; Sigma-Aldrich, St. Louis, MO) for 10 min at 37°C as described previously (Rogers et al., 2003). The cysts were blocked and permeabilized with PFS buffer for 30 min at room temperature, and stained with primary antibodies for 24 h and with secondary antibodies and phalloidin-rhodamine for 10–20 h at 4°C. All the antibodies were diluted in PFS buffer containing 0.02% NaN3. Cells or cysts were postfixed with 4% paraformaldehyde and mounted with mounting medium (Kirkegaard and Perry Laboratories, Gaithersburg, MD).
Images were acquired on a confocal microscope (LSM-510 META) with the accompanying software (both from Carl Zeiss, Thornwood, NY), by using a plan-Apochromat 100 × 1.40 numerical aperture oil differential interference contrast objective (Carl Zeiss) to detect Cy3 and Cy5 fluorochromes.
Electron Microscopy (EM)
Filter-grown cells were fixed in a solution containing 2% glutaraldehyde, 0.8% paraformaldehyde, and 0.1 M cacodylate. For transmission EM, the cells were stained with osmium and imidazole as described previously (Lipschutz et al., 2000), dehydrated, embedded in resin, sectioned, and imaged (JEOL 1010, fitted with a Hamamatsu digital camera and imaging software from Advanced Microscopy Techniques, Danvers, MA).
For scanning EM, the fixed cells were rinsed with 100 mM cacodylate buffer, dehydrated through a graded ethanol series, washed with hexamethyldisilazane (Electron Microscopy Sciences), dried for 5 min at 60°C, coated with platinum, and analyzed on a scanning EM machine (XL20 SEM; FEI Company, Hillsboro, OR).
Real-Time Polymerase Chain Reaction (RT-PCR)
Fifteen micrograms of total RNA collected from MDCK cells grown in 24-well plate were converted to first-strand cDNA. cDNA and the TaqMan primer/probe system, individualized for Sec8, Sec10, and Exo70, were used in conjunction with the 7700 Prism sequence detection instrument (both Applied Biosystems, Foster City, CA) as described in the Applied Biosystems technical manual. When the reaction product amplification exceeded the threshold value, the corresponding cycle number was termed CT. -Fold change between conditions was calculated through an exponential function of the observed difference in CT as described previously (Livak and Schmittgen, 2001). The values were normalized to a control mRNA, the 18S ribosome, and all real-time PCR studies were performed at least three times in triplicate.
Antibody Generation
Sec10 antibody was generated by injecting a C-terminal peptide, CAEQKKTDFKPEDENN, predicted to be highly antigenic using structure modeling, into rabbits by BioSource International (Camarillo, CA). The rabbits were bled, and the antibody was affinity purified according to standard protocols by BioSource International. The C-terminal Sec10 antibody worked well for Western blot (see Figures 1, 4, 6, and 7) but did not work for immunofluorescence (IF) or EM (data not shown). An N-terminal Sec10 antibody was also generated but did not work for either Western blot or IF (data not shown).
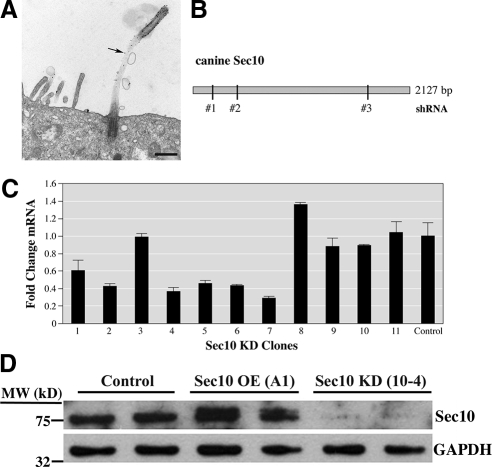
Figure 1.
Sec10 localizes to the cilia and shRNA against Sec10 results in decreased expression. (A) Sec10-myc expressing MDCK cells were grown on Transwell filters for 7 d. Pre-embedding immunolabeling was performed. Mouse anti-myc antibody was used at 1:200 dilution to identify the myc epitope present on Sec10, because antibody to Sec10 that works well with EM was not available to us. Secondary goat anti-mouse antibody was tagged with ultrasmall gold (Aurion, Immuno Gold Reagents & Accessories, Wageningen, The Netherlands). The gold label was further enhanced with silver staining for 25 min and formed black dots on the image (arrow). The image was taken on a FEI Tecnai transmission electron microscope (53,400× magnification). Bar, 0.5 μm. (B) Cartoon showing canine Sec10 and the regions of Sec10 that the shRNAs were designed against. (C) MDCK cells infected with a lentivirus expressed the Sec10 shRNA and a GFP reporter that allowed for FACS. After FACS single-cell sorting, clones were grown in 24-well dishes, split into multiple wells, and assayed for Sec10 mRNA expression. Several clones showed significantly decreased expression (60–80%) at the mRNA level, including clone 4 (hereafter referred to as 10-4). (D) Control MDCK, Sec10-overexpressing (OE) MDCK, and Sec10 knockdown (KD) MDCK cells were grown to confluence in 10-cm tissue culture dishes. Equal amounts of protein, as determined by bicinchoninic acid assay and Western blot of the housekeeping protein glyceraldehyde-3-phosphate dehydrogenase (GAPDH), were loaded in each well and Western blot by using our newly created antibody against Sec10 was performed. More Sec10 protein was seen in Sec10 OE cells and less protein was seen in Sec10 KD cells compared with control cells. Sec10 knockdown at the mRNA, and protein levels seemed to be similar.
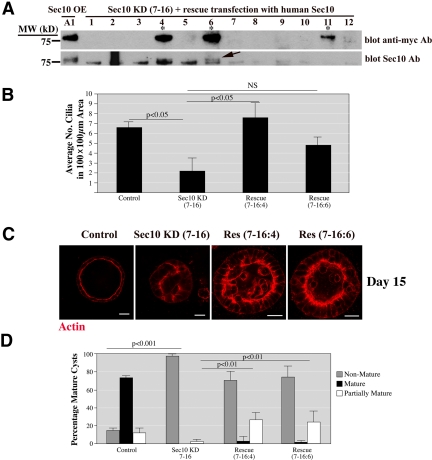
Figure 4.
Rescue of the ciliogenesis and cystogenesis defects by human Sec10. (A) Human Sec10 (hSec10) resistant to canine shRNA, by virtue of a different primary base pair structure, was transfected into the stable Sec10 shRNA KD cells. New stable cell lines were generated containing both Sec10 shRNA and hSec10-myc transcribed off the pcDNA3 vector. Using anti-myc antibody, three clonal cell lines, of 36 tested, were found to express hSec10-myc. These cell lines showed hSec10-myc expression at the same molecular weight as seen in the hSec10-overexpressing cell lines that we generated previously (Lipschutz et al., 2000) (*, 4, 6, and 11). For further confirmation, we used our newly generated Sec10 polyclonal antibody for Western blot. In the cell lines expressing hSec10-myc (4, 6, and 11), a double band can be seen (arrow). The top band is hSec10, which is slightly heavier because of the 10 extra amino acids in the myc epitope tag. 7-16 is a parent MDCK cell line (Sec10 shRNA 7, clone 16), in which Sec10 knockdown was obtained. “Rescue” clones were 7-16:4, 7-16:6, and 7-16:11, as determined by myc and Sec10 staining. (B) The number of primary cilia per 100- × 100-μm area was greater in the rescued cells compared with the Sec10 KD cells as determined by SEM. NS, nonsignificant. “Control” refers to normal MDCK cells. (C) Similarly, the rescued cells formed more cyst structures than did the knockdown cells at day 15 as seen by immunofluorescence staining of actin with phalloidin-rhodamine. The rescue with respect to cyst formation was partial, as the cysts from the rescued cells were not completely mature at day 15. Bar, 20 μm. (D) Quantification of the cyst rescue. We defined partial rescue as a cyst lumen area greater than 30% of the total area at the point of greatest cyst diameter.
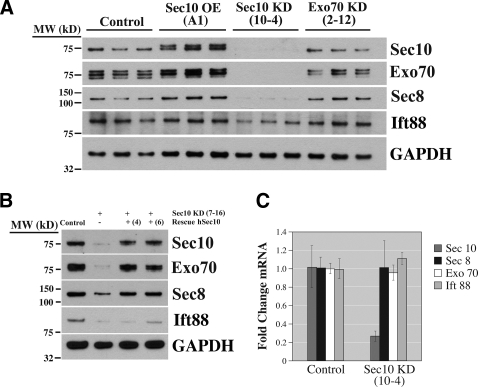
Figure 6.
Knockdown of Sec10 results in decreased expression of other exocyst proteins and Ift88. (A) Control, Sec10-overexpressing (OE), Sec10 knockdown (KD), and Exo70 KD cells were grown on Transwell filters for 14 d, and Western blot was performed on the cell lysate. Sec10 KD resulted in decreased protein expression of Sec10, Exo70, Sec8, and Ift88. In contrast, Exo70 KD did not affect Sec8, Sec10, or Ift88 protein expression. (B) Rescue of the Sec10 KD cells by transfection with human Sec10 resistant to canine shRNA, as described in Figure 4, resulted in restoration of Sec10, Exo70, Sec8, and, to a lesser degree, Ift88 protein levels to those seen in control cells. (C) To determine whether the decrease in Exo70, Sec8, and Ift88 protein levels was because of transcriptional inhibition, mRNA was obtained and RT-PCR performed. In the Sec10 KD cells, the mRNA expression levels for Exo70, Sec8, and Ift88 were similar to control levels, indicating that the decrease in Exo70, Sec8, and Ift88 protein expression in Sec10 KD cells was a posttranscriptional event.
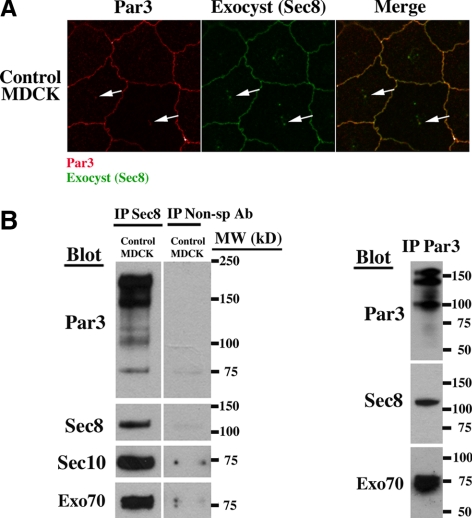
Figure 7.
The exocyst and Par3 colocalize and coimmunoprecipitate. (A) Par 3 was previously shown to be involved in primary cilia biogenesis (Sfakianos et al., 2007). To test for a possible exocyst–Par3 interaction, we performed double labeling immunofluorescence microscopy by using antibodies against Par3 and the exocyst component Sec8. Par3 and Sec8 colocalized at the tight junction and primary cilia (arrows) in control MDCK cells. (B) We next investigated a biochemical interaction by coimmunoprecipitation. Control MDCK cells were grown on Transwell filters for 14 d and the cells were lysed in a nonionic detergent (1% IGEPAL). IP was performed with antibody against Sec8 and the gel was then blotted with antibody against Par3, Sec8, Sec10, and Exo70. The presence of Par3, Sec10, and Exo70 bands after immunoprecipitation with Sec8 antibody demonstrates a biochemical interaction. The lanes are from the same gel; however, the control (nonspecific, anti-myc antibody) lane was separated on the gel from the other lanes shown. The reverse IP was performed with antibody against Par3 and the gel was blotted with antibody against Par3, Sec8, and Exo70, confirming a biochemical interaction.
Coimmunoprecipitation (CoIP) and Western Blotting
MDCK cells grown on the 10-cm dishes were collected on ice in a lysis buffer containing 20 mM HEPES, pH 7.4, 120 mM NaCl, 1 mM EDTA, 1% IGEPAL CA-630, 0.02% NaN3, 0.2% Trasylol, and proteinase inhibitor cocktail (1:1000) and then centrifuged at 14,000 rpm for 20 min at 4°C. The soluble supernatants were incubated overnight at 4°C with the indicated antibodies at a concentration of 1 μg/ml. Immunocomplexes were then precipitated with protein A-Sepharose 4B (GE Healthcare, Chalfont St. Giles, Buckinghamshire, United Kingdom). The immunocomplexes were washed five times with lysis buffer, eluted by boiling in SDS-polyacrylamide gel electrophoresis sample buffer, and then subjected to immunoblot analysis. To measure the interaction between Par3 and exocyst complex, Sec8 was precipitated with a monoclonal anti-Sec8 antibody (Stressgen Biotechnologies, Victoria, BC, Canada), the immunocomplex was blotted with a rabbit polyclonal IgG1 anti-Par3 antibody (Millipore, Billerica, MA). In a reciprocal experiment, Par3 was precipitated with the polyclonal anti-Par3 antibody, and the immunocomplex was blotted with the monoclonal anti-Sec8 antibody. Blots were developed by enhanced chemiluminescence (Pierce Chemical, Rockford, IL).
To measure the expression level of the targeted protein in the knockdown cell lines, the cells were lysed in 0.5% SDS lysis buffer (0.5% SDS, 100 mM NaCl, 50 mM tetraethylammonium-Cl, pH 8.1, 5 mM EDTA, 0.2% Trasylol, and 0.02% NaN3) and prepared in standard manner (Lipschutz et al., 2003). Antibody against Exo-70 was a kind gift from S. Hsu (Rutgers University, The State University of New Jersey-New Brunswick/Piscataway, NJ). Antibody against Ift88 was a kind gift from Brad Yoder (University of Alabama at Birmingham, Birmingham, AL).
Statistics
All graphs, with the exception of cilia length in Figure 2A, show mean plus SD. For measurement of cilia length the data were binned, because the cilia could be in different stages of development, and a nonparametric Kruskal–Wallis test was performed. For analysis of the ratio of cilia to nuclei, logistic regression was used. The calculations were performed by the Biostatistics Consulting Service of the University of Pennsylvania Cancer Center (Philadelphia, PA).
Figure 2.
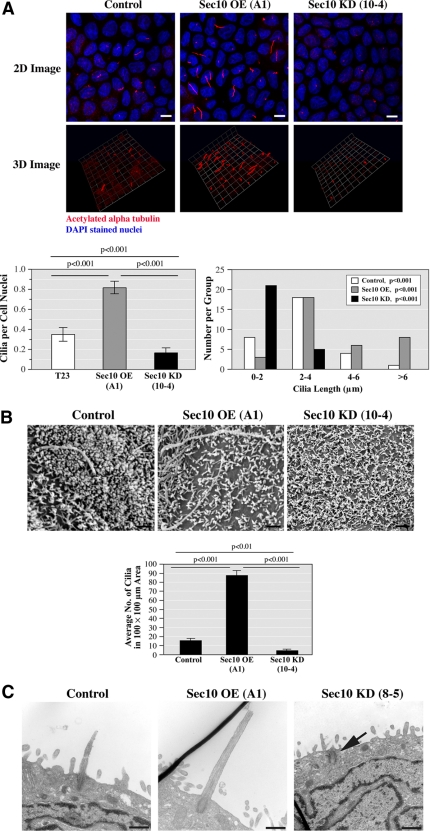
Sec10 knockdown leads to decreased primary ciliogenesis. (A) Control, Sec10-overexpressing (OE), and Sec10 knockdown (KD) MDCK cells were grown on Transwell filters for 14 d. Using antibody against acetylated α-tubulin that is specific for primary cilia, we examined ciliogenesis by immunofluorescence staining and confocal microscopy, as well as 3D reconstruction of the stacked series. Primary cilia were more abundant (as determined by the ratio of primary cilia/cell nuclei) and longer in Sec10 OE cells compared with control cells and less abundant and shorter in Sec10 KD cells compared with control cells. Quantification of ciliary abundance and cilia length are shown in the graphs below the images. Bar, 5 μm. The images are representative of >50 fields examined, with each experiment being repeated three times. Although MDCK cells are well ciliated and have long been used to study ciliogenesis (Praetorius and Spring, 2001), for unknown reasons cilia formation in MDCK cells is relatively sporadic, with the literature describing from ∼30% of normal MDCK cells being ciliated, as we see, to as little as 14.6% (Shalom et al., 2008). (B) To examine ciliogenesis by another method and in more detail, we grew control, Sec10 OE, and Sec10 KD cells on Transwell filters for 14 d. The cells were fixed in glutaraldehyde and SEM was performed (FEI Company XL20 SEM). A 200 mesh thinbar EM grid was placed on the samples and the number of cilia in a 100- × 100-μm area was determined. Confirming the results in Figure 2A, primary cilia seemed longer and therefore easily identifiable, in Sec10 OE cells compared with control cells and were rarely identified in Sec10 KD cells. Quantification of these results is shown on the graph below the images. Bar, 0.5 μm. (C) Using TEM, images of the cilia and basal bodies were occasionally captured. Sec10 KD cells demonstrated only basal bodies (arrow). In contrast, the control and Sec10 OE cells showed both basal bodies and elongated cilia, with the length of the cilia greater in the Sec10 OE cells. Bar, 0.5 μm.
RESULTS
Generation of Exocyst Knockdown Cell Lines
We previously showed by immunofluorescence that the exocyst complex localized, to the tight junction and primary cilia (and/or basal body) of MDCK cells (Rogers et al., 2004). To confirm this and to examine the ciliary localization of the exocyst in greater detail, we used electron gold microscopy. In Sec10-myc–overexpressing cells, the Sec10 component of the exocyst localized along the entire length of the primary cilia (Figure 1A), raising the possibility that it could be involved in ciliary protein delivery and biogenesis.
To investigate the role of the exocyst in ciliogenesis, we decided to knockdown exocyst expression in MDCK cells by using an shRNA lentiviral vector delivery system. We focused our efforts on Sec10 because we had shown previously, by overexpression, that Sec10 was centrally involved in cystogenesis (Lipschutz et al., 2000).
Three shRNA oligos were created for Sec10 by using the Elledge Lab pPRIME system (Stegmeier et al., 2005) (Figure 1B) (see Materials and Methods for shRNA sequence and details). The shRNA sequences were cloned into the p199 cloning vector and then into a lentiviral delivery system for infection into MDCK cells. The p199 vector encodes GFP, which allowed us to identify and separate the infected MDCK cells by using fluorescence-activated cell sorting (FACS). Significant knockdown of Sec10 was confirmed at the mRNA level (Figure 1C). Because Sec10 antibodies were not commercially available, we generated a rabbit polyclonal antibody using a C-terminal peptide (see Materials and Methods for details). This antibody worked well for Western blot, and a similarly significant knockdown of Sec10 at the protein level was seen (Figure 1D).
Sec10 Knockdown Results in Decreased Primary Ciliogenesis
To examine the role of the exocyst in cilia biogenesis, we performed immunofluorescence staining in the control, Sec10-overexpressing, and Sec10 knockdown MDCK cells grown for 2 wk on Transwell filters. By immunofluorescence and 3D reconstruction, there was significantly greater ciliary elongation in the Sec10-overexpressing compared with control cells, and a significant decrease in cilia length in the Sec10 knockdown cells. In addition, the ratio of cilia to nuclei was significantly increased in the Sec10-overexpressing compared with control cells and significantly reduced in the Sec10 knockdown compared with control cells (Figure 2A). To confirm the above-mentioned results, we performed scanning electron microscopy (SEM). SEM showed significantly fewer elongated and therefore identifiable cilia present per unit area in the Sec10 knockdown cells compared with control cells, and significantly more cilia in the Sec10-overexpressing cells (Figure 2B).
To further examine cilia, morphology in the Sec10 mutant cells, transmission electron microscopy (TEM) was performed. Although it was difficult to capture images of the cilia and basal bodies by thin section TEM, the Sec10 knockdown cells demonstrated mainly basal bodies (Figure 2C, arrow). In contrast, TEM using the control and Sec10-overexpressing cells showed basal bodies and elongated cilia. Although statistics could not be performed because of the paucity of images, the cilia in the Sec10-overexpressing cells seemed longer than in control cells (Figure 2C). Similar results were seen using other MDCK Sec10 mutant cell lines (data not shown).
To investigate Sec10 knockdown in another ciliated cell line, the spontaneously arising retinal pigment epithelia cell line ARPE-19 was used (den Hollander et al., 2007). Stable cell lines were generated as described above, and a similar disruption of ciliogenesis was seen (Supplemental Figure 1).
Sec10 Knockdown Inhibits Normal MDCK Cyst Morphogenesis
As discussed, MDCK cells form hollow monoclonal cysts when placed in a collagen matrix (Hellman et al., 2008), and primary cilia seem to be centrally involved in renal cystic disease (Smyth et al., 2003). To examine the role of Sec10 in cystogenesis, control, Sec10-overexpressing, and Sec10 knockdown MDCK cells were seeded at low (single-cell) density in a collagen matrix. Control cells formed typical hollow cysts as described previously (Lipschutz et al., 2000). Sec10-overexpressing cells underwent cyst morphogenesis at a faster rate, whereas Sec10 knockdown cells did not form hollow mature cysts but instead formed irregular clumps of cells lacking a lumen (Figure 3).
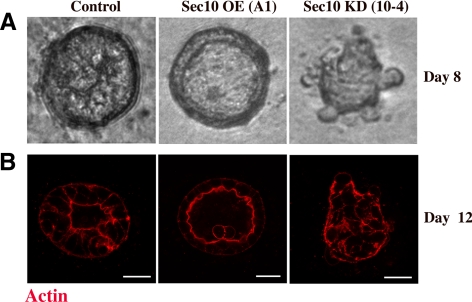
Figure 3.
Sec10 knockdown prevents MDCK cystogenesis. A) Control, Sec10-overexpressing (OE), and Sec10 knockdown (KD) MDCK cells were seeded at low (single-cell) density in a collagen matrix. By 8 d, the Sec10 OE cells had already formed mature cysts, with a single lumen. Imaging of live cysts was done using Nomarski optics. (B) At 12 d, fixation and immunofluorescence staining of actin with phalloidin-rhodamine, showed maturing cysts in the control cells, mature cysts in the Sec10 OE cells, and immature cysts or clumps of cells in the Sec10 KD cells. Bar, 20 μm. Fully mature control cysts are seen in the majority of cases by 15 d (see Figure 4C).
Rescue with Human Sec10 Resistant to the shRNA Reverses the Phenotype
To confirm the specificity of the knockdown phenotype, we performed a rescue experiment using human Sec10 (hSec10) that is resistant to the canine shRNA by virtue of a difference in primary base pair structure. Stable cell lines were generated by transfecting hSec10 into the canine Sec10 shRNA knockdown cells. Three cell lines containing both knockdown of native canine Sec10 and overexpression of hSec10-myc were generated (Figure 4A).
Sec10 control, knockdown, and rescue cell lines were grown on Transwell filters for 15 d, and the cilia were examined by SEM. In the rescued cell lines, elongated cilia were identified at equivalent levels to control cells and higher levels than found in the parent Sec10 knockdown cells (Figure 4B).
Phenotypic rescue, with respect to cyst formation, was only partially successful in that cysts with multiple lumens formed. This may be because human and canine Sec10 are only 95% identical. Nevertheless, there was clearly rescue after transfection with hSec10 (Figure 4, C and D).
Because Sec10 and Sec15 are the most vesicle-proximal components of the exocyst and physically link the exocyst to vesicles carrying proteins (Guo et al., 1999), we were interested in seeing whether knockdown of other exocyst components would affect primary ciliogenesis and/or cystogenesis. Six shRNA oligos were created for Sec8 and Exo70 (3 each). MDCK cell lines with knockdown of Sec8 and Exo70 expression at the mRNA and protein levels were generated using identical methods described in Figure 1 (Supplemental Figure 2). Knockdown of the representative exocyst components Sec8 and Exo70 did not inhibit either primary ciliogenesis or cystogenesis (data not shown), although it should be emphasized that these are knockdown and not knockout experiments, and a role for Sec8 and Exo70 in these processes is still possible.
The Mechanism Does Not Seem to Be Because of a Defect in Cell Polarity
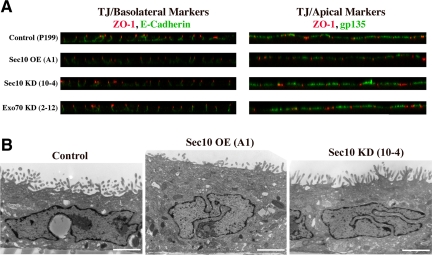
Given the importance of the exocyst in cell polarity, we first investigated whether the defect in ciliogenesis in Sec10 knockdown cells was because of abnormal cell polarity. Control, Sec10-overexpressing, Sec10 knockdown, and Exo70 knockdown MDCK cells were grown for 2 wk on Transwell filters. The cells were then stained for apical gp135, basolateral E-cadherin, and the tight junction protein zona occludens (ZO)-1. Cell polarity was not grossly affected as demonstrated by IF using basolateral, tight junction, and apical markers (Figure 5A). Similarly, low-magnification TEM showed no gross differences in cell structure (Figure 5B). Although we could not examine cell polarity in collagen because the Sec10 knockdown cells did not form cysts (Figure 3), it should be noted that cell polarity can be different in cells grown on two-dimensional filters compared with cells grown in three-dimensional collagen (O'Brien et al., 2001).
Figure 5.
Knockdown of Sec10 does not affect cell polarity. (A) Control, Sec10-overexpressing (OE), Sec10 knockdown (KD), and Exo70 KD cells showed grossly normal distribution of apical membrane (gp135), tight junction (ZO-1), and basolateral membrane (E-cadherin) proteins by confocal immunofluorescence staining. Please note that these cells express GFP off the shRNA vector, so Cy3 and Cy5 were used as fluorochromes. Cy5 was changed to green in these images to enhance visualization. (B) TEM microscopy also demonstrated normal cell polarity. We reported previously hat Sec10 OE cells were taller, most likely due to increased basolateral membrane delivery (Lipschutz et al., 2000, 2003), which we confirm here. Bar, 2 μm.
Knockdown of Sec10 Affects Protein Levels of Other Exocyst Components and Ift88
Knockdown of Sec10 resulted in reduced protein levels of Sec8 and Exo70. This was in contrast to knockdown of Sec8 and Exo70, where only Sec8 and Exo70, respectively, showed reduced protein expression. In addition, in the Sec10 knockdown cells the intraflagellar transport protein 88 (Ift88) was also decreased compared with control cells (Figure 6A).
Importantly, rescue of the canine shRNA with human Sec10 normalized the Sec8 and Exo70 protein levels, and increased levels of Ift88, providing further evidence that Sec10 knockdown specifically causes decreased expression of Sec8, Exo70, and Ift88 (Figure 6B).
To determine whether the reduction of Sec8, Exo70, and Ift88 in the Sec10 knockdown cells was because of a transcriptional event, we performed real-time PCR from the mRNA extract of the stable Sec10 knockdown cells. The mRNA expression of Sec8, Exo70, and Ift88 was similar in the control and knockdown cells, indicating that the lower Sec8, Exo70, and Ift88 protein levels seen in Sec10 knockdown cells were not a result of transcriptional repression (Figure 6C).
The Exocyst and Par3 Colocalize and Coimmunoprecipitate
It was recently shown that the PDZ protein Par3 localizes to the tight junction and primary cilium and knockdown of Par3 inhibits primary ciliogenesis (Sfakianos et al., 2007). Given a similar defect in ciliogenesis in the Sec10 knockdown cells (Figure 2), along with our previous data showing that the exocyst also localized to the tight junction and primary cilia (Rogers et al., 2004), we first confirmed that Par3 localizes to the primary cilium (Supplemental Figure 3). We then investigated a possible interaction by performing immunofluorescence double labeling. This showed that the exocyst and Par3 colocalized in an identical manner at both the tight junction and primary cilium (Figure 7A).
To further investigate an interaction, we performed coIP. We first immunoprecipitated the exocyst by using Sec8 antibody, which works well for coIP (Lipschutz et al., 2003); blotted for Par3; and then did the reverse experiment, immunoprecipitating with Par3 and blotting for the exocyst. These experiments demonstrate that Par3 and the exocyst specifically associate, though the interaction may not be direct (Figure 7B).
DISCUSSION
We report three principal findings here, all of which are quite interesting. First, we show that the exocyst, specifically the Sec10 component, is necessary for primary cilia biogenesis in MDCK renal tubular epithelial cells. This is especially interesting in that the exocyst was shown previously, in most studies, to be involved in basolateral, and not apical, membrane trafficking (Grindstaff et al., 1998; Lipschutz et al., 2000, 2003). These data add to a growing body of evidence suggesting that the primary cilium, whereas it is found on the apical surface, it is not a typical “apical organelle” and helps explain the fact that primary cilia contain many proteins that also localize to the basolateral membrane, including: α3β1 integrin (Praetorius et al., 2004), galectin (Bao and Hughes, 1999), and the PKD proteins polycystin-1, polycystin-2, and fibrocystin (Wang et al., 2007). In yeast, Sec4, a small Rab GTPase, is found on vesicles carrying polarized proteins and regulates the exocyst complex via interactions with Sec10/Sec15 (Guo et al., 1999). The closest mammalian homologues to Sec4 are Rab8, Rab10, and Rab13 (Pereira-Leal and Seabra, 2000), and knockdown of Rab8, which is also involved in basolateral trafficking, has been shown to inhibit ciliogenesis (Nachury et al., 2007; Yoshimura et al., 2007). Furthermore, knockdown of a guanine nucleotide exchange factor for Rab8, Rabin8, also inhibited ciliogenesis (Nachury et al., 2007). Other evidence supporting the idea that the primary cilium is a distinct organelle, neither apical nor basolateral, is that the ciliary membrane has a highly condensed bilayer domain at its base that could function as a fence to separate the ciliary membrane from the surrounding apical membrane (Vieira et al., 2006).
The second important finding is that Sec10 is a crucial component of the exocyst complex. The exocyst has classically been thought of as a complex, in which all eight proteins function together as a single unit (Lipschutz and Mostov, 2002). Here, however, Sec10 knockdown and overexpression had distinct effects on cilia biogenesis and cyst morphogenesis, whereas knockdown of other exocyst components did not affect either cilia biogenesis or cyst morphogenesis. Nevertheless, as noted, these results should be interpreted with caution because shRNA knocks down, but does not knock out, protein expression; therefore, a role for Sec8 and Exo70 in ciliogenesis or cystogenesis cannot be excluded.
Importantly, in this study when Sec10 was knocked down, several other exocyst components and the intraflagellar transport protein Ift88 were found to have lower protein expression levels because of a posttranscriptional event. It is tempting to speculate that this is because of increased proteosome degradation as the exocyst complex, minus Sec10, becomes unstable and disintegrates. Similarly, although there is no evidence to date that intraflagellar transport proteins, other than Ift20 (Follit et al., 2006), traffic in vesicles to the primary cilium; assuming this were the case, it is plausible that Ift88-positive vesicles would be degraded when they could no longer dock at the cilia/basal body because of loss of the exocyst complex. In any event, these data indicate that Sec10 is a central and particularly important component of the exocyst complex, at least with respect to primary cilia biogenesis. Although inhibition of ciliogenesis and the defect in cyst morphogenesis cannot be conclusively linked, it is interesting to note that MDCK cells overexpressing vesicular-integral membrane protein 17, a protein not known to be involved in basolateral transport, have short to absent cilia and are similarly unable to form normal cysts when placed in a collagen matrix (Takiar and Caplan, unpublished observations).
Finally, we show that the exocyst colocalizes with the PDZ protein Par3 at the tight junction and primary cilium and coimmunoprecipitates with Par3. Par3, as discussed, was recently shown to be necessary for the biogenesis of primary renal cilia (Sfakianos et al., 2007). Given the known role of the exocyst in polarized membrane trafficking, our data support a model in which the exocyst is localized to the primary cilium, perhaps by a small GTPase, and then binds to Par3 and/or a member of the Par complex, such as the transmembrane protein Crb3A. Once localized to the primary cilium, the exocyst targets and docks vesicles carrying ciliary proteins.
In summary, we have shown that the Sec10 component of the exocyst is involved in primary cilia biogenesis and cyst morphogenesis, most likely through interactions with Par3. Given the role of the primary cilium in cystic kidney disease, the exocyst may represent a novel therapeutic target.
Supplementary Material
ACKNOWLEDGMENTS
The University of Pennsylvania Biomedical Imaging Core Facility of the Cancer Center is gratefully acknowledged for providing confocal and EM imaging services, the Morphology Core of the Center for the Molecular Studies of Liver and Digestive Diseases (Center Grant P30 DK50306) for providing microscopy services, and the Biomedical Art and Design Core of the Biochemistry and Biophysics Department for providing assistance with preparation of the figures. We also acknowledge the Penn Vector Core at the University of Pennsylvania for providing vector production services and partial funding for the vectors through the Penn Vector Core NIDDK P30 Molecular Therapy Center grant. This work was supported in part by National Institutes of Health grants DK-069909 and DK-070980 (to J.H.L.) and GM-64690 (to W. G).
Footnotes
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E08-07-0772) on March 18, 2009.
REFERENCES
- Bao Q., Hughes R. C. Galectin-3 and polarized growth within collagen gels of wild-type and ricin-resistant MDCK renal epithelial cells. Glycobiology. 1999;9:489–495. doi: 10.1093/glycob/9.5.489. [DOI] [PubMed] [Google Scholar]
- Charron A. J., Bacallao R. L., Wandinger-Ness A. ADPKD: a human disease altering Golgi function and basolateral exocytosis in renal epithelia. Traffic. 2000;1:675–686. doi: 10.1034/j.1600-0854.2000.010811.x. [DOI] [PubMed] [Google Scholar]
- den Hollander A. I., et al. Mutations in LCA5, encoding the ciliary protein lebercilin, cause Leber congenital amaurosis. Nat. Genet. 2007;39:889–895. doi: 10.1038/ng2066. [DOI] [PubMed] [Google Scholar]
- Fan S., Hurd T. W., Liu C. J., Straight S. W., Weimbs T., Hurd E. A., Domino S. E., Margolis B. Polarity proteins control ciliogenesis via kinesin motor interactions. Curr. Biol. 2004;14:1451–1461. doi: 10.1016/j.cub.2004.08.025. [DOI] [PubMed] [Google Scholar]
- Fawcett D. W., Porter K. R. A study of the fine structure of ciliated epithelia. J. Morphol. 1954;94:221–281. [Google Scholar]
- Finger F. P., Novick P. Spatial regulation of exocytosis: lessons from yeast. J. Cell Biol. 1998;142:609–612. doi: 10.1083/jcb.142.3.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Follit J. A., Tuft R. A., Fogarty K. E., Pazour G. J. The intraflagellar transport protein IFT20 is associated with the Golgi complex and is required for cilia assembly. Mol. Biol. Cell. 2006;17:3781–3792. doi: 10.1091/mbc.E06-02-0133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grindstaff K. K., Yeaman C., Anandasabapathy N., Hsu S., Rodriguez-Boulan R., Scheller R. H., Nelson W. J. Sec6/8 complex is recruited to cell-cell contacts and specifies transport vesicle delivery to the basal-lateral membrane in epithelial cells. Cell. 1998;93:731–740. doi: 10.1016/s0092-8674(00)81435-x. [DOI] [PubMed] [Google Scholar]
- Guo W., Roth D., Walch-Solimena C., Novick P. The exocyst is an effector for Sec4p, targeting secretory vesicles to sites of exocytosis. EMBO J. 1999;18:1071–1080. doi: 10.1093/emboj/18.4.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellman N. E., Spector J., Robinson J., Zuo X., Saunier S., Antignac C., Tobias J. W., Lipschutz J. H. Matrix metalloproteinase 13 (MMP13) and tissue inhibitor of matrix metalloproteinase 1 (TIMP1), regulated by the MAPK pathway, are both necessary for Madin-Darby canine kidney tubulogenesis. J. Biol. Chem. 2008;283:4272–4282. doi: 10.1074/jbc.M708027200. [DOI] [PubMed] [Google Scholar]
- Hsu S., Ting A. E., Hazuka C. D., Davanger S., Kenny J. W., Kee Y., Scheller R. H. The mammalian brain rsec6/8 complex. Neuron. 1996;17:1209–1219. doi: 10.1016/s0896-6273(00)80251-2. [DOI] [PubMed] [Google Scholar]
- Lipschutz J. H., Guo W., O'Brien L. E., Nguyen Y. H., Novick P., Mostov K. E. Exocyst is involved in cystogenesis and tubulogenesis and acts by modulating synthesis and delivery of basolateral plasma membrane and secretory proteins. Mol. Biol. Cell. 2000;11:4259–4275. doi: 10.1091/mbc.11.12.4259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipschutz J. H., Lingappa V. R., Mostov K. E. The exocyst affects protein synthesis by acting on the translocation machinery of the endoplasmic reticulum. J. Biol. Chem. 2003;278:20954–20960. doi: 10.1074/jbc.M213210200. [DOI] [PubMed] [Google Scholar]
- Lipschutz J. H., Mostov K. E. The many masters of the exocyst. Curr. Biol. 2002;12:R212–R214. doi: 10.1016/s0960-9822(02)00753-4. [DOI] [PubMed] [Google Scholar]
- Liu Q., Tan G., Levenkova N., Li T., Pugh E. N., Jr, Rux J. J., Speicher D. W., Pierce E. A. The proteome of the mouse photoreceptor sensory cilium complex. Mol. Cell Proteomics. 2007;6:1299–1317. doi: 10.1074/mcp.M700054-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak K. J., Schmittgen T. D. Analysis of relative gene expression data using real-time quantitative PCR and the 2 (-delta, deltaCT) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Nachury M. V., et al. A core complex of BBS proteins cooperates with the GTPase Rab8 to promote ciliary membrane biogenesis. Cell. 2007;129:1201–1213. doi: 10.1016/j.cell.2007.03.053. [DOI] [PubMed] [Google Scholar]
- Novick P., Field C., Schekman R. Identification of 23 complementation groups required for post-translational events in the yeast secretory pathway. Cell. 1980;21:205–221. doi: 10.1016/0092-8674(80)90128-2. [DOI] [PubMed] [Google Scholar]
- O'Brien L. E., Jou T. S., Pollack A. L., Zhang Q., Hansen S. H., Yurchenco P., Mostov K. E. Rac1 orientates epithelial apical polarity through effects on basolateral laminin assembly. Nat. Cell Biol. 2001;3:831–838. doi: 10.1038/ncb0901-831. [DOI] [PubMed] [Google Scholar]
- Pereira-Leal J. B., Seabra M. C. The mammalian Rab family of small GTPases: definition of family and subfamily sequence motifs suggests a mechanism for functional specificity in the Ras superfamily. J. Mol. Biol. 2000;301:1077–1087. doi: 10.1006/jmbi.2000.4010. [DOI] [PubMed] [Google Scholar]
- Praetorius H. A., Praetorius J., Nielsen S., Frokiaer J., Spring K. R. Beta1-integrins in the primary cilium of MDCK cells potentiate fibronectin-induced Ca2+ signaling. Am. J. Physiol. Renal Physiol. 2004;287:F969–F978. doi: 10.1152/ajprenal.00096.2004. [DOI] [PubMed] [Google Scholar]
- Praetorius H. A., Spring K. R. Bending the MDCK cell primary cilium increases intracellular calcium. J. Membr. Biol. 2001;184:71–79. doi: 10.1007/s00232-001-0075-4. [DOI] [PubMed] [Google Scholar]
- Rogers K. K., Jou T. S., Guo W., Lipschutz J. H. The Rho family of small GTPases is involved in epithelial cystogenesis and tubulogenesis. Kidney Int. 2003;63:1632–1644. doi: 10.1046/j.1523-1755.2003.00902.x. [DOI] [PubMed] [Google Scholar]
- Rogers K. K., Wilson P. D., Zhang X., Guo W., Burrow C. R., Lipschutz J. H. The exocyst localizes to the primary cilium in MDCK cells. Biochem. Biophys. Res. Commun. 2004;319:138–143. doi: 10.1016/j.bbrc.2004.04.165. [DOI] [PubMed] [Google Scholar]
- Sfakianos J., Togawa A., Maday S., Hull M., Pypaert M., Cantley L., Toomre D., Mellman I. Par3 functions in the biogenesis of the primary cilium in polarized epithelial cells. J. Cell Biol. 2007;179:1133–1140. doi: 10.1083/jcb.200709111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shalom O., Shalva N., Altschuler Y., Motro B. The mammalian Nek1 kinase is involved in primary cilium formation. FEBS Lett. 2008;582:1465–1470. doi: 10.1016/j.febslet.2008.03.036. [DOI] [PubMed] [Google Scholar]
- Smyth B. J., Snyder R., Balkovetz D. F., Lipschutz J. H. Recent advances in the cell biology of polycystic kidney disease. In: Jeon K. W., editor. International Review of Cytology. vol. 231. San Diego, CA: Elsevier; 2003. pp. 52–89. [DOI] [PubMed] [Google Scholar]
- Stegmeier F., Hu G., Rickles R. J., Hannon G. J., Elledge S. J. A lentiviral microRNA-based system for single-copy polymerase II-regulated RNA interference in mammalian cells. Proc. Natl. Acad. Sci. USA. 2005;102:13212–13217. doi: 10.1073/pnas.0506306102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terbush D. R., Maurice T., Roth D., Novick P. The exocyst is a multiprotein complex required for exocytosis in Saccharomyces cerevisiae. EMBO J. 1996;15:6483–6494. [PMC free article] [PubMed] [Google Scholar]
- Vieira O. V., Gaus K., Verkade P., Fullekrug J., Vaz W. L., Simons K. FAPP2, cilium formation, and compartmentalization of the apical membrane in polarized Madin-Darby canine kidney (MDCK) cells. Proc. Natl. Acad. Sci. USA. 2006;103:18556–18561. doi: 10.1073/pnas.0608291103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S., Zhang J., Nauli S. M., Li X., Starremans P. G., Luo Y., Roberts K. A., Zhou J. Fibrocystin/polyductin, found in the same protein complex with polycystin-2, regulates calcium responses in kidney epithelia. Mol. Cell Biol. 2007;27:3241–3252. doi: 10.1128/MCB.00072-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webber W. A., Lee J. Fine structure of mammalian renal cilia. Anat. Rec. 1975;182:339–343. doi: 10.1002/ar.1091820307. [DOI] [PubMed] [Google Scholar]
- Yoshimura S., Egerer J., Fuchs E., Haas A. K., Barr F. A. Functional dissection of Rab GTPases involved in primary cilium formation. J. Cell Biol. 2007;178:363–369. doi: 10.1083/jcb.200703047. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.