Abstract
Background
Hostility and anger have been attributed as psychosocial risk factors for coronary heart disease. Heightened cardiovascular reactivity (CVR), and poor recovery, to provocative stressors is thought to hasten this risk.
Purpose
To examine the relationship between hostility and anger inhibition (AI), and the moderating situational influences of harassment and evaluation, in predicting CVR and recovery to mental arithmetic (MA) stress using a multiple regression approach.
Methods
48 male undergraduate students engaged in the following 3 minute tasks during recording of the electrocardiogram, impedance cardiography, and blood pressure: baseline, MA, and evaluation. Hostility and AI were assessed with the Cook-Medley Hostility Scale and the Speilberger Anger In subscale, respectively.
Results
An interaction between hostility and AI showed high diastolic blood pressure reactivity to the MA task among hostile anger inhibitors. Harassment did not modify this effect. However, harasser evaluation predicted prolonged systolic blood pressure (SBP) responding among men scoring high in AI, and facilitated SBP recovery among those scoring low on AI.
Conclusions
The findings highlight the interactive influences of AI and hostility in predicting CVR to stress and underscore the importance of recovery assessments in understanding the potentially pathogenic associations of these constructs.
Keywords: Hostility, Anger-In, Harassment, Evaluation, Cardiovascular Reactivity, Recovery
Dispositional hostility and anger have been attributed as psychosocial risk factors for coronary heart disease (CHD) (e.g., Everson-Rose & Lewis, 2005; Miller et al., 1996; Sirois & Burg, 2003). Hostile people are prone to cynical attitudes and a mistrust of others, which may give rise to the frequent experience of anger and various associated behaviors. Situations requiring anger inhibition may be more prevalent in the daily life experiences of hostile individuals than encounters permitting anger expression (Brosschot & Thayer, 1998). Moreover, the tendency to suppress anger has been linked to more pronounced carotid arterial stiffness and intima-medial thickness, sub-clinical indices of CHD, compared to individuals rating high on anger expression (Anderson et al., 2006). Some evidence suggests that hostile persons who inhibit their anger expression are more likely to develop significant coronary atherosclerosis than hostile individuals who express their anger (e.g., Atchison & Condon, 1993; Dembroski et al., 1985; Matthews et al., 1998).
Hostile individuals have been found to display pronounced cardiovascular reactivity (CVR) to stressors involving interpersonal provocation or harassment relative to their non-hostile counterparts (e.g., Davis et al., 2000; Suarez et al., 1998; Suls & Wan, 1993). Insofar as these stress responses are frequent and large in magnitude, they are thought to contribute to pathogenic processes linked to CHD risk (e.g., Kop, 1999). However, some reports indicate that hostile individuals may not display significant CVR to stressors involving harassment or anger recall, but rather show prolonged CV responses to such stressors that are reflected in poor recovery to baseline following stressor completion (e.g., Anderson et al., 2005; Neumann et al., 2004), whereas other reports have found hostile individuals to display both pronounced CVR and poor recovery from stressors involving anger elicitation (e.g., Fredrickson et al., 2000).
These findings are in accord with the prolonged activation-perseverative cognition hypothesis, by which the tendency to worry or ruminate may prolong stress responding, and in so doing serve as a final common pathway by which stress exerts deleterious effects on bodily systems and health (Brosschot et al., 2006). In fact, worry and rumination have been linked to a variety of negative CV characteristics such as delayed blood pressure (BP) recovery to stress (Gerin et al., 2006; Glynn et al., 2002), and elevated heart rate (HR) and reduced HR variability (Brosschot et al., 2007; Hofmann et al., 2005; Knepp & Friedman, 2008; Pieper et al., 2007; Thayer et al., 1996). Moreover, longitudinal data indicate that high trait worry may confer increased CHD risk in men (Kubzansky, et al., 1997). Anger suppression may act similarly to worry by maintaining awareness of negative cognitions.
Tendencies toward anger inhibition, as assessed via the defensiveness construct by use of the Marlowe-Crowne Social Desirability Scale (MC; Crowne & Marlow, 1964), have been associated with elevated CVR to mental arithmetic (MA) stress when combined with high levels of hostility (Jorgenson et al., 1995; Larson & Langer, 1997). However, inconsistencies have persisted in the defensive hostility literature, whereby defensively hostile individuals have displayed CVR to stressors similar in magnitude to individuals rating low on these constructs (Mente & Helmers, 1999; Shapiro et al., 1995; Vella & Friedman, 2007). Another study found hostile individuals to display significant systolic blood pressure (SBP) reactivity to an interpersonally provoking debate task, but non-significant interactions between hostility and defensiveness in predicting CVR (Powch & Houston, 1996). One potential explanation for these discrepancies concerns the notion that the MC scale assesses behaviors unrelated to the suppression of angry feelings. A more direct measure of anger inhibition may be preferred and can be achieved with the anger-in (AI) subscale from the Spielberger Anger Expression Scale (Spielberger et al., 1985).
Evidence suggests that hostility may interact with AI scores to predict elevations in sympathetic β-adrenergic influences on the heart, as evidenced by decreases in impedance cardiography derived pre-ejection period (PEP) and decreased inter-beat intervals (IBI), in response to MA stress (Burns et al., 1992). However, individuals rating low on both of these scales also displayed significant reductions in PEP to the MA task, which could be due to the absence of interpersonal provocation in the stressor (e.g., Suls & Wan, 1993).
In addition to the potentially critical moderating influence of harassment in the relationship between hostility and CV responses to stress, assessments of the ability to evaluate the source of anger provocation may provide insight into another situational influence that modifies the recovery process. The inability to express anger following provocation among hostile individuals may attenuate CV recovery compared to those rating low on hostility, a tendency that may be accompanied by low cardiac vagal activity (Brosschot & Thayer, 1998). A ‘matching hypothesis’ has been proposed to explain findings in which use of one’s preferred mode of anger management style facilitates CV recovery from stress (Engebretson et al., 1989). The idea behind this hypothesis concerns a ‘person-environment’ fit, such that individuals rating high on AI may show facilitated BP recovery when instructed to write a positive evaluation of an experimenter following harassment-induced stress, but poor recovery when told to write a negative evaluation of the experimenter after stressor completion.
The concept of a general ‘person-environment’ fit theory has a longstanding history in social psychology (e.g, Lewin, 1951), with qualities reflected in the transactional model of stress (Lazarus & Folkman, 1984). Support for such matching hypotheses of person-environment fit has been reported with respect to interactions between measures of interpersonal style and situational characteristics in predicting cardiovascular responses to stress (e.g., Davis & Matthews, 1996; Smith & Ruiz, 2007). However, a previous attempt to replicate the matching hypothesis concerning anger management style found no support for this ‘person-environment’ fit (Lai & Linden, 1992). A plausible explanation for this null finding is the need to directly consider the role of hostility in this relationship. A test of the matching hypothesis might reveal hostile individuals scoring low on AI to benefit from the influence of provocateur evaluation on CV recovery, whereas hostile individuals scoring high on AI display a prolonged activation that persists after evaluation of a provocateur. The combination of hostility with anger inhibition on a person or situation level (i.e., AI or the inability to evaluate the source of provocation following harassment), may be linked to enduring hostile cognitions reflected in a delayed return of cardiac vagal activity and slow CV recovery (Brosschot & Thayer, 1998).
The present study examines the interaction between hostility and AI on CV responses to MA stress with or without harassment, in addition to the influence of experimenter evaluation on CV recovery. The combination of hostility and AI may be associated with stressor-induced CVR, poor CV recovery from stress, and potentially stress-related CHD. Men generally have shown greater CV reactivity to lab and field stressors relative to women (e.g., Guyll & Contrada, 1998; Stoney, 1992). To control for gender, only male subjects were included in the present study. Hostile men rating high in AI were expected to show the most CVR to harassment- induced MA stress, in addition to poor CV recovery. In accord with the matching hypothesis, hostile men rating low on AI were expected to show enhanced CV recovery when given the opportunity to evaluate their provocateur, whereas experimenter evaluation was predicted to be associated with weak CV recovery among individuals rating high on both hostility and AI. This study adds to the literature by testing the interaction between hostility and AI in predicting the CVR to stress with harassment, in addition to assessing the influence of evaluation on CV recovery.
Method
Participants
Forty eight healthy male undergraduate psychology students (M = 19.38, SD = 1.67 yrs; range: 18–27 yrs) at Virginia Polytechnic Institute & State University (Virginia Tech) were recruited from on-line advertisements posted on their Psychology Department Experiment Management System. This study received approval from the institutional review board at Virginia Tech. The sample consisted of individuals of Caucasian (85.4%), Asian American (10.4%), and African American (4.2%) ethnicities and roughly approximated the Virginia Tech population base rates. Participants were selected on the basis of information obtained from a health questionnaire. Exclusionary criteria included a positive smoking status and/or use of medications that may alter CV activity. Participants were instructed to abstain from caffeine for 12 hrs and alcohol for 24 hrs prior to the study and received extra credit in a psychology course for their participation. Sample characteristics are displayed in Table 1.
Table 1.
Sample Characteristics and Cardiovascular Responses to Mental Arithmetic Stress
| Characteristic | M | SD | |||
|---|---|---|---|---|---|
| Age (years) | 19.38 | 1.67 | |||
| Body Mass Index (kg/m2) | 23.77 | 3.33 | |||
| % Non-Caucasian Ethnicity | 14.6 | ||||
| Caffeine intake (8 oz drinks/day) | 1.33 | 1.4 | |||
| Alcohol intake (drinks/week) | 6.83 | 8.36 | |||
| Cook-Medley Hostility Scale | 22.02 | 7.64 | |||
| Spielberger Anger-In Scale | 16.33 | 4.40 | |||
| Baseline | Task | ||||
| Cardiovascular Measure | M | SD | M | SD | |
|
| |||||
| Heart Rate (bpm) | 69.35 | 10.5 | 86.67 | 12.99** | |
| Systolic Blood Pressure (mmHg) | 119.81 | 10.79 | 135.32 | 13.66** | |
| Diastolic Blood Pressure (mmHg) | 68.48 | 8.86 | 79.72 | 11.06** | |
| Pre-ejection Period (ms) | 121.02 | 16.2 | 111.64 | 19.84** | |
| Log High Frequency (ms2 Hz−1) | 13.78 | .926 | 13.2 | .98** | |
| LF/HF Ratio (normalized units) | 2.11 | 1.65 | 2.74 | 1.63† | |
Note. N = 48.
p <.06.
p <.001.
Apparatus
The Cook-Medley Hostility Scale (CMHS; Cook & Medley, 1954) was used to assess dispositional hostility in the current study and consists of 50 true-false items from the Minnesota Multi-phasic Personality Inventory (Hathaway & McKinley, 1943). In combined samples of more than 600 men and 600 women, high levels of internal consistency has been determined for the CMHS, with Cronbach’s alphas ranging from .80 to .82 for both men and women (Smith & Frohm, 1985). The test-retest correlations are high (r >.8) over periods of 1–4 years (Barefoot et al., 1983; Schekelle et al., 1983). Example items include, “It is safer to trust nobody,” “I am not easily angered,” (reverse scored) and “I have at times had to be rough with people who were rude or annoying.”
Anger-in was assessed by the 8 item AI subscale of the Spielberger Anger Expression Inventory (Spielberger et al., 1985). Items from this subscale measure how often angry feelings are experienced but not expressed. Sample items include, “I withdraw from people” and “I tend to harbor grudges that I don’t tell anyone about”. Participants responded to each item by indicating how often they generally reacted or behaved accordingly on a four-point scale (1 = almost never, 4 = almost always). Adequate internal consistency for the AI subscale (α = .74) has been found in a sample of 266 college aged men (Spielberger, 1999). In addition to the AI subscale, participants also completed the 8 item Anger-out (AO) subscale from the Spielberger Anger Expression Inventory (Spielberger et al., 1985). Items from this subscale measure how often angry feelings are expressed in verbally or physically aggressive behaviors, using the same self report 4 point scale as the AI subscale. Sample items include, “I say nasty things” and “I strike out at whatever infuriates me.” The AO subscale has been found to display adequate internal consistency (α = .78) amid a sample of 262 college aged men (Spielberger, 1999).
State anger was measured with the S-Anger subscale of the State-Trait Anger Expression Inventory (Spielberger, 1999). The 15 items of the subscale are rated on 4 point Likert scales and are summed with higher scores indicating greater anger. Internal consistencies for this subscale have been observed to range from .92–.95 in samples of healthy adults (Spielberger, 1999).
The electrocardiogram (ECG) and impedence cardiography (ICG) was recorded with the Ambulatory Monitoring System (AMS) v 4.4 (Vrije Universiteit Amsterdam, the Netherlands), using Ag-AgCl electrodes; the validity and reliability of this device has been established (Willemsen et al., 1996). BP was monitored by use of an IBS SD-700A monitor (Industrial & Biomedical Sensors Corp., Waltham, MA).
Dependent Variables
The measure derived from the ECG was HR, expressed in cardiac IBI. The ECG was analog filtered (high pass 17 Hz) at acquisition and subjected to online auto trigger level R-wave detection resulting in a heart period resolution of 1 ms. The ECG IBI data were analyzed with an autoregressive spectral estimation method (Matlab, v.7.4, 2004), which has some advantages over the traditional fast Fourier transform methods (Task Force, 1996), such as ease of satisfying the condition of stationarity for short time series. IBI’s represent the amount of time in milliseconds that elapses between the R spikes from the cardiac waveform. The differences between adjacent IBI values were computed and subjected to an ordinary least squares regression procedure for detrending. A natural logarithm (ln) procedure was employed (SPSS, v.15, 2007) to correct for skewed raw score distributions in the spectral data. Output residuals were used to create power spectral density units (ms2 Hz−1). Low frequency (LF; 0.04–0.15 Hz) and high frequency (HF; 0.15–0.40 Hz) ranges were extracted from the power spectral density units. The HF component serves as a measure of cardiac vagal activity (Akeselrod et al., 1981; Pomeranz et al., 1985), whereas the LF component has been argued to reflect fluctuations of sympathetic influences on cardiac dynamics (Malliani et al., 1991; Pagani et al., 1986). However, some have questioned the use of LF power as an index of sympathetic β adrenergic activation (Porges, 2007; Eckberg, 2000). Since LF power can be influenced by parasympathetic activity, an index of sympathovagal influences on the heart was computed with the LF/HF ratio, with normalized units of spectral estimates used in the final analysis as a reliable measure of autonomic balance (see Malliani, 1999). Higher ratios indicate increases in sympathetic β adrenergic activity and imply reductions in vagal control of HR.
Impedance cardiography provided a measure of PEP, an index of β-adrenergic influence on myocardial contractility (Sherwood et al., 1992). Systolic and diastolic BP was assessed via the oscillometric method with the automated IBS-SD 700A monitor. A microphone inside the cuff detected Korotkoff sounds from the brachial artery and provided digital displays of BP measures every 90 seconds for recording.
Procedure
All participants signed an electronic version of the informed consent for the present study and completed the health screening information form, in addition to the AI, AO, and CMHS scales, on-line. Upon arrival at the lab, each participant signed an additional copy of the informed consent, completed the state anger scale, and had six thoracic electrodes applied to the torso to record ECG and ICG in accord with configuration guidelines described in the AMS user manual v 1.2 (Vrije Universiteit, Amsterdam, the Netherlands). The BP cuff was placed on the dominant arm.
All task instructions were given to the participants prior to each task administration. Participants engaged in the following 3-minute laboratory tasks:
Baseline (BL): The participant was instructed to sit quietly and relaxed in a comfortable lounge chair. This procedure served as a resting baseline.
Mental Arithmetic (MA): The participant was instructed to engage in a serial subtraction task by counting backward out loud by 7’s from 2000. This challenging task has been known to elicit sympathetic β-adrenergic activity and parasympathetic withdrawal (Obrist, 1981). All subjects were instructed to perform as accurately and as fast as possible during this task. Performance was monitored for accuracy to ensure that subjects were engaged and trying to complete the task.
Evaluation: In the experimental condition, participants were given a questionnaire with items asking them to evaluate the experimenters and procedures in the current study. A similar questionnaire was used in a previous investigation employing comparable procedures (Lai & Linden, 1992). In the control condition, participants were given a questionnaire with items asking them to evaluate the psychology course for which they received extra credit through participation. Following completion of the evaluation period, participants repeated the state anger scale.
Harassment Manipulation
To test the effects of harassment on CVR, subjects performed the MA task under conditions involving either harassment or no harassment. Subjects were exposed to verbal harassment through tape-recorded statements, ostensibly coming from a research technician, played over an intercom. Three statements were used as harassing prods and were played at 45 second intervals irrespective to performance: 1)“You’re making too many mistakes, so try harder”; 2)“You’re still too slow and inaccurate, so focus”; and 3) “This can’t be the best you can do. You’re not trying hard enough”. The harassing statements were made by a man who used a stern, emphatic tone of voice. This method of MA harassment was first established by Hokanson and Shelter (1961) and has since been used in a variety of studies assessing anger relevant traits and CVR and recovery (e.g., Lai & Linden, 1992). Subjects in the non-harassment condition were permitted to complete the task without commentary.
Statistical Analyses
The effects of psychological traits (i.e., hostility and AI) and task manipulations (i.e., harassment and evaluation) on CV changes were evaluated with multiple regression analyses (SPSS, v 15, 2007). Testing interactive models in this fashion permits determination of whether experimental manipulations moderate the influence of traits, and/or trait X trait interactions, on CV reactivity and recovery (see Baron & Kenny, 1986). Simple slopes analyses were conducted as post hoc tests to probe and interpret interaction effects (Cohen, Cohen, West, & Aiken, 2003). To control for Type 1 error rate, a ‘protected t test approach’ was used, whereby t tests corresponding to individual predictor variables in regression models were evaluated for significance only in the context of a significant omnibus F test. For each model, body mass index (BMI) and race were entered as fixed effects covariates. Since there were few non-Asian minorities in the sample (n = 2), race was coded as a binary variable (0 = Caucasian, 1 = Minority). Anger-out scores were entered as a fixed effects covariate for all models involving AI, to provide more precision in the prediction of AI on CV responding to the study procedures. Harassment and evaluation were entered into the regression models using a dummy coding scheme, in which non-harassment and teacher evaluation were used as comparison groups, respectively (Cohen et al., 2003). Casewise diagnostics were run on each regression model to identify and withhold outliers, defined as outside 3 standard deviations from the mean. Finally, to address the issue of multicollinearity and ensure the interpretability of the observed interactions, bivariate correlations between predictor variables were observed to determine values <.8, and the collinearity diagnostics feature was used in SPSS, to verify all variance inflation factors of predictor variables were less than the recommended cut-off value of 4 (see Hutcheson & Sofroniou, 1999).
Arithmetic change scores (MA – BL) were calculated to index CVR for each dependent variable (DV). Recovery change scores were calculated for each DV as well (evaluation – BL). Simple difference values were used to assess reactivity and recovery rather than residualized change scores since: 1) the former is more readily interpretable, 2) the two methods have been found to be equally generalizable across tasks, and 3) difference scores have been found to be fairly reliable in reactivity research (Llabre et al., 1991).1
Cardiovascular Reactivity
The first set of regression models tested the main effects of harassment, hostility, and AI, in addition to the interactions among these variables, on CVR change scores for each DV. Predictor variables were entered into the regression models in a hierarchical step fashion. Two way interaction effects were tested for Harassment X Hostility, Harassment X AI, and Hostility X AI, followed by a test of the three way interaction term, Harassment X Hostility X AI.
Cardiovascular Recovery
A second set of regression models tested the significance of main effects and interaction terms among harassment, evaluation type, hostility, and AI in predicting CV recovery during the evaluation period. The same two-way interaction terms were tested as for CVR, with the addition of the harassment X evaluation term. The three-way interactions on CV recovery change scores included Harassment X Hostility X AI, Harassment X Evaluation X Hostility, and Harassment X Evaluation X AI. Finally, the four way interaction term was tested. Interaction terms were computed by multiplying the relevant variables.
Results
Preliminary Analyses
Baseline CV measures did not vary as a function of hostility or AI. Repeated measures ANOVA’s showed significant changes from baseline to MA task on all CV measures except the LF/HF ratio, for which there was a marginal effect: increases were observed for HR, F(1, 46) = 166.43, p < .001; SBP, F(1, 46) = 117.78, p < .001; DBP, F(1, 46) = 79.55, p < .001; and LF/HF ratio, F(1, 46) = 3.85, p = .056; and decreases were observed for PEP, F(1, 46) = 30.71, p < .001; and HF, F(1, 46) = 16.33, p < .001 (see Table 1). Intercorrelations for hostility, AI, CVR, and CV recovery are displayed in Table 2. Anger-out scores correlated positively with hostility (r = .45, p = .002) and was marginally linked to AI (r = .24, p = .1).
Table 2.
Intercorrelations of Trait Scales, Cardiovascular Reactivity (1), and Cardiovascular Recovery (2)
| Measure | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. | Hostility | -- | .59** | .16 | .15 | .15 | .22 | −.02 | −.004 | .15 | .16 | −.22 | −.17 | .06 | −.05 |
| 2. | Anger-In | -- | .10 | .23 | .03 | .06 | .13 | −.21 | .09 | .07 | −.07 | −.003 | .06 | .18 | |
| 3. | HR1 | -- | .47** | .55** | .28† | −.03 | .04 | −.53** | −.09 | .26† | −.03 | −.50** | −.16 | ||
| 4. | HR2 | -- | .19 | .36* | .12 | .12 | −.42** | −.42** | .24† | .22 | −.02 | .21 | |||
| 5. | SBP1 | -- | .53** | .15 | .24† | −.24† | −.03 | .06 | −.14 | −.35* | −.47** | ||||
| 6. | SBP2 | -- | −.02 | −.01 | −.12 | −.07 | −.18 | −.11 | −.22 | −.20 | |||||
| 7. | DBP1 | -- | .06 | −.04 | .08 | −.07 | −.25† | .04 | .04 | ||||||
| 8. | DBP2 | -- | −.01 | .15 | −.03 | −.01 | .09 | −.44** | |||||||
| 9. | HF-HRV1 | -- | .66** | −.50** | −.22 | .21 | −.08 | ||||||||
| 10. | HF-HRV2 | -- | −.31* | −.44** | .16 | −.36* | |||||||||
| 11. | LF/HF1 | -- | .61** | −.04 | .21 | ||||||||||
| 12. | LF/HF2 | -- | .10 | .26† | |||||||||||
| 13. | PEP1 | -- | .11 | ||||||||||||
| 14. | PEP2 | -- | |||||||||||||
Note. N = 48.
p <.1.
p <.05.
p <.01.
HR = Heart Rate. SBP = Systolic Blood Pressure. DBP = Diastolic Blood Pressure. HF-HRV = High Frequency Heart Rate Variability. LF/HF = Low-High Frequency Ratio. PEP = Pre-ejection Period.
State Anger
A paired samples t-test on Spielberger State Anger scores revealed MA stress to be associated with significant increases in anger, t(47) = 3.157, p < .003 (M = 15.67, SD = 1.68 pre stressor; M = 16.44, SD = 2.47 post stressor). Further, regression analyses revealed hostility to be positively associated with baseline (B = .102, SE = .029; t = 3.574, p = .001; R2 = .217) and post stressor (B = .124, SE = .044; t = 2.825, p = .007; R2 = .148) state anger scores. However, an independent samples t-test revealed a non-significant effect for harassment on state anger scores, t(45) =1.272, p = .210. Hostility did not interact with harassment in predicting state anger.
Cardiovascular Reactivity
Multiple regression analyses were conducted on HR, SBP, DBP, PEP, HF power, and the LF/HF ratio reactivity change scores, with BMI and race entered in each model as between subject covariates, and AO scores entered as a between subject covariate for all models involving AI. Initial analyses on between subject covariates revealed race to predict DBP reactivity to MA stress (B = 8.31, SE = 3.30; t = 2.515, p = .016; R2 = .180), indicating individuals of Asian or African American ethnicities to display larger increases in DBP to MA stress relative to Caucasian participants. No main effects were observed for harassment, hostility, or AI on the reactivity change scores.
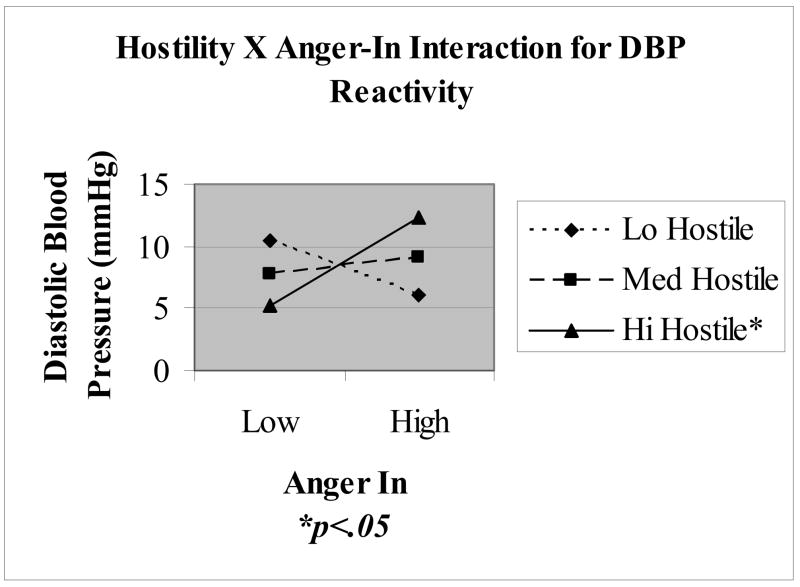
A significant interaction was observed between hostility and AI in predicting DBP reactivity (B = .082, SE = .032; t = 2. 6, p = .013; R2 = .322, ΔR2 = .111), as well as a marginal interaction between these variables for SBP reactivity (B = .068, SE = .041; t = 1.68, p = .1; R2 = .142, ΔR2 = .06). Figure 1 displays predicted DBP reactivity values based upon a simple slopes analysis at one standard deviation (SD) above and below the centered means for CMHS and AI. Results indicated that AI moderated the effects of hostility on DBP responses to MA stress, whereby hostile men scoring high on AI displayed the most task reactivity (12.47 mmHg), which was significantly greater than hostile men scoring low on AI (5.74 mmHg). Although simple slopes analyses for the Hostility X AI interaction of SBP reactivity were non-significant, the pattern was similar to that observed with DBP, whereby hostile anger inhibitors evidenced the most pronounced SBP reactivity to MA stress. No other significant reactivity interactions were noted, including the three way Harassment X Hostility X AI term.
Figure 1.
Interaction between hostility and anger in on diastolic blood pressure reactivity to mental arithmetic stress. Simple slopes reflect predicted values at 1 standard deviation above and below the centered mean for hostility and anger in.
Recovery Scores
A marginal main effect was observed for harassment on LF/HF ratio recovery change scores during the evaluation period (B = −1.13, SE = .568; t = −1.99, p = .052; R2 = .103, ΔR2 = .081), indicating harassment to be associated with reductions in LF power during the evaluation period. However, this main effect is qualified by a significant Harassment X AI interaction (B =−0.254, SE = .099; t = −2.56, p = .014; R2 = .306, ΔR2 = .114) for the LF/HF ratio. Simple slopes analysis was significant for individuals rating high on AI, indicating LF power suppression (smaller ratios relative to baseline) following MA stress with harassment and prolonged LF power responding (higher ratios relative to baseline) to MA stress without harassment. LF/HF recovery ratios did not vary as a function of harassment for individuals rating low in AI.
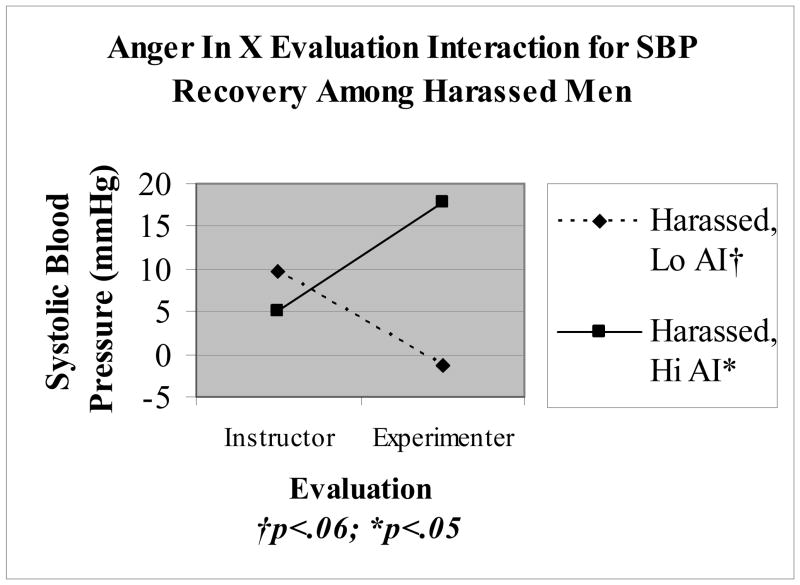
No main effects for hostility or AI were observed on CV recovery change scores during the evaluation period. Significant 3 way interactions were observed for Harassment X Evaluation X AI (B = 3.27, SE = 1.28; t = 2.56, p = .015; R2 = .323, ΔR2 = .119) and Harassment X Evaluation X Hostility (B = 1.83, SE = .814; t = 2.24, p = .031; R2 = .279, ΔR2 = .095) on SBP recovery during the evaluation period. Follow up analyses indicated the interaction between evaluation and AI to be significant among harassed participants (B = 2.7, SE = .917; t = 2.95, p = .005), whereas this interaction in the absence of harassment was non-significant, p >.5. Figure 2 displays predicted SBP values at 1 SD above and below the centered AI mean for the Evaluation X AI interaction. Consistent with the matching hypothesis, harassed men rating high in AI showed attenuated SBP recovery during experimenter evaluation relative to instructor evaluation, whereas harassed men scoring low on AI showed an opposite effect with facilitated SBP recovery during experimenter evaluation relative to instructor evaluation.
Figure 2.
Interaction between evaluation and anger in on systolic blood pressure recovery values among harassed participants. Simple slopes reflect predicted values at 1 standard deviation above and below the centered mean for anger in.
Follow up analyses for the Harassment X Evaluation X Hostility interaction for SBP recovery revealed a non-significant interaction between evaluation and CMHS among harassed participants, B = −.855, p = .187. No other significant interactions were observed for the evaluation period, including the 4 way interaction.
Discussion
The aim of the current study was to examine the relationship between hostility and AI, and the potentially moderating situational influences of harassment and evaluation in predicting CVR and recovery to MA stress. The findings regarding the first hypothesis of hostility interacting with AI levels to predict exaggerated CV responses to harassment induced stress were mixed. Consistent with the defensive hostility literature (Jorgenson et al., 1995; Larson & Langer, 1997), men scoring high in hostility and AI were found to display the most pronounced DBP reactivity to MA stress (see Figure 1). However, this effect was not found to be modified by the influence of harassment. The current findings suggest that the MA task alone is a potent and frustrating stressor, producing reliable increases in sympathetic α- and β-adrenergic measures and decreases in cardiac vagal activity (see Table 1), accompanied by increases in state anger. Indeed, MA itself may be experienced as intrinsically harassing, and so the harassment manipulation may not have added substantial perceived stress. Although hostility was found to predict pre and post stressor state anger scores, the harassment manipulation was not found to predict state anger, nor did hostility interact with harassment to predict state anger.
It is notable that harassment interacted with AI to predict LF/HF ratio recovery during the evaluation period. Contrary to prediction, individuals scoring high on AI showed prolonged stress responding during the evaluation period following MA stress without provocation. These findings appear to suggest that the harassment manipulation was not effective at eliciting sustained increases in sympathetic β-adrenergic responding in men scoring high in AI.
The second aim of the current study was to test the ‘matching hypothesis’, to determine whether assessment of ‘person-environment’ fit is instructive in predicting CV recovery from stress. Specifically, hostile men scoring low on AI were predicted to benefit from provocateur evaluation, whereas such evaluation was predicted to be associated with attenuated recovery among men scoring high on both hostility and AI. Partial support was found for this hypothesis in the current study, whereby men scoring high in AI showed attenuated SBP recovery particular to experimenter evaluation (see Figure 2). Although the interaction between evaluation and hostility was non-significant for harassed participants, the pattern of SBP responding suggests a similar relationship to that observed with AI. These results are consistent with Engebretson et al. (1989) and suggest that anger management style may map on to situational characteristics surrounding life stressors to predict CV recovery.
Another finding of interest concerns the elevated DBP reactivity among individuals of Asian or African American ethnicities relative to Caucasian individuals. Ethnic disparities in BP reactivity to stress have been well documented (e.g., Barnes et al., 2000; Wilcox et al., 2005). However, the bulk of research has focused on differences between Caucasian and African Americans, with a relative dearth on Asian ethnicities. There is some evidence to suggest that individuals of Asian ethnicities may exhibit lower CVR to stress compared to Caucasian individuals (e.g., Shen et al., 2004; Stoney et al., 2002). The ethnic distribution in the current study did not permit comparisons to be made among different minorities. Future studies may take these issues into account when examining more diversified samples.
Although the current study contributes to the literature by providing concurrent assessments of hostility and AI, in addition to considering the situational influence of provocateur evaluation on CV recovery, a few key limitations should be noted. First, the current sample size may have restricted the ability to detect significant effects based upon predicted interactive relationships. However, the results were in at least partial support of the current hypotheses, whereby hostile anger inhibitors displayed the most pronounced DBP reactivity to MA stress, and harassed men rating high in AI showed the weakest SBP recovery following provocateur evaluation. Nonetheless, the potential issue of being underpowered for some of the tests examined cannot be completely ruled out and so remains a limitation that may account for the mixed pattern in the current findings, which should be interpreted with caution and merit replication in a larger sample.
Second, since hostile individuals are prone to a cynical mistrust of others, they may have been suspicious of the harassment manipulation. Prerecorded prods were administered via intercom in the current study for standardization purposes, but may have seemed too artificial to be effective. Other studies that reported significant results in CV responses related to harassment induced MA stress personalized the prods by having the harasser say the participant’s name as part of the procedure, or asked the participant to start from the beginning with each interruption (e.g., Anderson et al., 2005; Glynn et al., 2002; Lai & Linden, 1992). Further, the 15 item Spielberger State Anger Scale may have been too lengthy and narrow to broadly detect mood changes as a manipulation check in the current study. Harassment can produce increases in anger, fear, disgust, and sadness, as well as decreases in happiness, as has been shown by the use of a briefer but more varied state emotion inventory as manipulation check (Anderson, Linden, & Habra, 2006). Future steps to ensure the veracity of the harassment manipulation will include the use of a more efficient manipulation check, personalization of harassment prods, and having participants meet the harasser at some point prior to beginning the task.
An alternative possibility is that under non-harassment, high AI individuals may have ruminated and felt more distress about their performance during the recovery period. In contrast, when such individuals had been harassed and were also hostile, they may have been able to avoid negative feelings about their performance by focusing on the provocateur. Although plausible, this interpretation of the AI X harassment interaction for LF/HF ratio conflicts with the finding that harassed men scoring high in AI showed delayed SBP recovery following provocateur evaluation.
Finally, the present findings were drawn from a sample of young, healthy adult men of a predominantly Caucasian background (85%), limiting the generalizability of the results. Previous research has revealed women to be more prone to AI (e.g., Houston & Vavak, 1991) and this tendency to be associated with DBP reactivity to stress among women relative to men (e.g., Harralson et al., 1997). Other studies have suggested African American men rating high in AI to show enhanced CV reactivity to lab stress (Finney et al., 2002) and ambulatory blood pressure responses to daily stressors (Brownley et al., 1996) compared to Caucasian men. The present sample of individuals from ethnic minorities was too small to permit testing interaction effects involving ethnicity. To the extent that focusing on gender and ethnicity informs a greater understanding of CVR to stress and disease associations related to hostility and anger, future studies may consider a more diversified sample.
The main findings of this study suggest that hostility may interact with AI in predicting stress related CVR. Consistent with previous research, the influence of harassment on CV responses to MA stress among hostile individuals may be most apparent with respect to prolonged BP responding following stressor completion (Anderson et al., 2005). Further, AI was found to interact with situational influences following harassment induced stress, providing support for the matching hypothesis of a ‘person-environment’ fit when predicting CV recovery, which has been reported previously (Engebretson et al., 1989). Taken together, the current findings point toward the importance of considering interactive relationships when predicting CV responses to stress among hostile populations. Hostile anger inhibitors may be prone to pronounced DBP responses to stressors, and situational influences may moderate CV recovery based upon anger management style. These findings complement a prolonged activation-perseverative cognition hypothesis (Brosschot et al., 2006; Gerin et al., 2006), whereby ruminative tendencies interact with situational influences to prolong stress responding in a way that may precipitate CV disease development and progression. Future studies will take necessary measures to ensure the verisimilitude of the harassment manipulation, in addition to including gender and ethnicity as key variables of interest in a larger sample.
Acknowledgments
These data were collected in the Department of Psychology at Virginia Tech. The authors would like to thank Jeffrey Elcano, Thomas Evans, and Michael Sweet for assistance in data collection, and Israel Christie for technical assistance. Portions of these data were presented at the annual meetings of the Society for Psychophysiological Research (2005), the Society for Behavioral Medicine (2006), and the Association for Psychological Science (2006).
Footnotes
Baseline values of cardiovascular variables were uncorrelated with reactivity change scores, and controlling for baseline levels in these variables did not change the statistical significance nor the nature of the findings in terms of reactivity or recovery.
This research was supported in part by the National Institute of Health Training Grant HL07560 awarded to Dr. Vella.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Akselrod S, Gordon E, Ubel FA, Shannon DC, Barger AC, Cohen RJ. Power spectrum analysis of heart rate fluctuations: A quantitative probe of beat-to-beat cardiovascular control. Science. 1981;213:220–222. doi: 10.1126/science.6166045. [DOI] [PubMed] [Google Scholar]
- Anderson JC, Linden W, Habra ME. The importance of examining blood pressure reactivity and recovery in anger provocation research. International Journal of Psychophysiology. 2005;57:159–163. doi: 10.1016/j.ijpsycho.2004.12.011. [DOI] [PubMed] [Google Scholar]
- Anderson JC, Linden W, Habra ME. Influence of apologies and trait hostility on recovery from anger. Journal of Behavioral Medicine. 2006;29(4):347–358. doi: 10.1007/s10865-006-9062-7. [DOI] [PubMed] [Google Scholar]
- Anderson DE, Metter EJ, Hougaku H, Najjar SS. Suppressed anger is associated with increased carotid arterial stiffness in older adults. American Journal of Hypertension. 2006;19:1129–1134. doi: 10.1016/j.amjhyper.2006.04.018. [DOI] [PubMed] [Google Scholar]
- Atchison M, Condon J. Hostility and anger measures in coronary heart disease. Australian and New Zealand Journal of Psychiatry. 1993;27(3):436–442. doi: 10.3109/00048679309075800. [DOI] [PubMed] [Google Scholar]
- Barefoot JC, Dahlstrom G, Williams RB. Coronary heart disease incidence and total mortality: A 25 year follow-up study of 255 physicians. Psychosomatic Medicine. 1983;45:59–63. doi: 10.1097/00006842-198303000-00008. [DOI] [PubMed] [Google Scholar]
- Barnes VA, Trieber FA, Musante L, Turner JR, Davis H, Strong WB. Ethnicity and socioeconomic status: Impact on cardiovascular activity at rest and during stress in youth with a family history of hypertension. Ethnicity and Disease. 2000;10(1):4–16. [PubMed] [Google Scholar]
- Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: Conceptual, strategic, and statistical considerations. Journal of Personality and Social Psychology. 1986;51:1173–1182. doi: 10.1037//0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- Brosschot JF, Gerin W, Thayer JF. The perseverative cognition hypothesis: A review of worry, prolonged stress-related physiological activation, and health. Journal of Psychosomatic Research. 2006;60:113–124. doi: 10.1016/j.jpsychores.2005.06.074. [DOI] [PubMed] [Google Scholar]
- Brosschot JF, Van Dijk E, Thayer JF. Daily worry is related to low heart rate variability during waking and the subsequent nocturnal sleep period. International Journal of Psychophysiology. 2007;63:39–47. doi: 10.1016/j.ijpsycho.2006.07.016. [DOI] [PubMed] [Google Scholar]
- Brosschot JF, Thayer JF. Anger inhibition, cardiovascular recovery, and vagal function: a model of the link between hostility and cardiovascular disease. Annals of Behavioral Medicine. 1998;20(4):326–32. doi: 10.1007/BF02886382. [DOI] [PubMed] [Google Scholar]
- Brownley KA, Light KC, Anderson NB. Social support and hostility interact to influence clinic, work, and home blood pressure in Black and White men and women. Psychophysiology. 1996;33:434–445. doi: 10.1111/j.1469-8986.1996.tb01069.x. [DOI] [PubMed] [Google Scholar]
- Burns JW, Friedman R, Katkin ES. Anger expression, hostility, anxiety, and patterns of cardiac reactivity to stress. Behavioral Medicine. 1992;18:71–78. doi: 10.1080/08964289.1992.9935174. [DOI] [PubMed] [Google Scholar]
- Cohen J, Cohen P, West SG, Aiken LS. Applied Multiple Regression/Correlation Analysis for the Behavioral Sciences. 3. Mahwah, NJ: Lawrence Erlbaum Associates; 2003. [Google Scholar]
- Cook WW, Medley DM. Proposed hostility and pharisaic-virtue scales for the MMPI. Journal of Applied Psychology. 1954;238:414–18. [Google Scholar]
- Crowne DP, Marlowe D. The Approval Motive: Studies in Evaluative Dependence. New York: Wiley; 1964. [Google Scholar]
- Davis MC, Matthews KA. Do gender-relevant characteristics determine cardiovascular reactivity? Match versus mismatch of traits and situation. Journal of Personality and Social Psychology. 1996;71(3):527–535. doi: 10.1037//0022-3514.71.3.527. [DOI] [PubMed] [Google Scholar]
- Davis MC, Matthews KA, McGrath CE. Hostile attitudes predict elevated vascular resistance during interpersonal stress in men and women. Psychosomatic Medicine. 2000;62:17–25. doi: 10.1097/00006842-200001000-00003. [DOI] [PubMed] [Google Scholar]
- Dembroski TM, MacDougal JM, Williams RB, Haney TL, Blumenthal JA. Components of Type A, hostility, and anger-in: Relationship to angiographic findings. Psychosomatic Medicine. 1985;47(3):219–233. doi: 10.1097/00006842-198505000-00001. [DOI] [PubMed] [Google Scholar]
- Eckberg DL. Physiological basis of human autonomic rhythms. Annals of Medicine. 2000;32(5):341–349. doi: 10.3109/07853890008995937. [DOI] [PubMed] [Google Scholar]
- Engebretson TO, Matthews KA, Scheier MF. Relations between anger expression and cardiovascular reactivity: Reconciling inconsistent findings through a matching hypothesis. Journal of Personality and Social Psychology. 1989;57:513–521. doi: 10.1037//0022-3514.57.3.513. [DOI] [PubMed] [Google Scholar]
- Everson-Rose SA, Lewis TT. Psychosocial factors and cardiovascular disease. Annual Review of Public Health. 2005;26:469–500. doi: 10.1146/annurev.publhealth.26.021304.144542. [DOI] [PubMed] [Google Scholar]
- Finney ML, Stoney CM, Engebretson TO. Hostility and anger expression in African American and European American men is associated with cardiovascular and lipid reactivity. Psychophysiology. 2002;39:340–349. doi: 10.1017/s0048577201393101. [DOI] [PubMed] [Google Scholar]
- Fredrickson BL, Maynard KE, Helens MJ, Haney TL, Siegler IC, Barefoot JC. Hostility predicts magnitude and duration of BP response to anger. Journal of Behavioral Medicine. 2000;23(3):229–243. doi: 10.1023/a:1005596208324. [DOI] [PubMed] [Google Scholar]
- Gerin W, Davidson KW, Chirstenfeld NJS, Goyal T, Schwartz JE. The role of angry rumination and distraction in blood pressure recovery from emotional arousal. Psychosomatic Medicine. 2006;68:64–72. doi: 10.1097/01.psy.0000195747.12404.aa. [DOI] [PubMed] [Google Scholar]
- Glynn LM, Christenfeld N, Gerin W. The role of rumination in recovery from reactivity: Cardiovascular consequences of emotional states. Psychosomatic Medicine. 2002;63:714–726. doi: 10.1097/01.psy.0000031574.42041.23. [DOI] [PubMed] [Google Scholar]
- Guyll M, Contrada RJ. Trait hostility and ambulatory cardiovascular activity: responses to social interaction. Health Psychology. 1998;17:30–39. doi: 10.1037//0278-6133.17.1.30. [DOI] [PubMed] [Google Scholar]
- Harralson TL, Suarez EC, Lawler KA. Cardiovascular reactivity among hostile men and women: The effects of sex and anger suppression. Women’s Health. 1997;3(2):151–164. [PubMed] [Google Scholar]
- Hathaway SR, McKinley JC. Booklet for the Minnesota Multiphasic Personality Inventory. New York: The Psychological Corporation; 1943. [Google Scholar]
- Hofmann SG, Moscovitch DA, Litz BT, Kim H-J, Davis LL, Pizzagalli DA. The worried mind: Autonomic and prefrontal activation during worrying. Emotion. 2005;5:464–475. doi: 10.1037/1528-3542.5.4.464. [DOI] [PubMed] [Google Scholar]
- Hokanson J, Shelter S. The effects of overt aggression on physiological arousal level. Journal of Abnormal Social Psychology. 1961;63:446–448. doi: 10.1037/h0046864. [DOI] [PubMed] [Google Scholar]
- Houston BK, Vavak CR. Cynical hostility: Developmental factors, psychosocial correlates, and health behaviors. Health Psychology. 1991;10(1):9–17. doi: 10.1037//0278-6133.10.1.9. [DOI] [PubMed] [Google Scholar]
- Hutcheson G, Sofroniou N. The Multivariate Social Scientist: Introductory Statistics using General Linear Models. Thousand Oaks, CA: Sage Publications; 1999. [Google Scholar]
- Jorgenson RS, Abdul-Karim K, Kahan TA, Frankowski JJ. Defensiveness, cynical hostility and cardiovascular reactivity: A moderator analysis. Psychotherapy and Psychosomatics. 1995;64:156–161. doi: 10.1159/000289006. [DOI] [PubMed] [Google Scholar]
- Knepp MM, Friedman BH. Cardiovascular activity during laboratory tasks in women with high and low worry. Biological Psychology. 2008;79:287–293. doi: 10.1016/j.biopsycho.2008.07.002. [DOI] [PubMed] [Google Scholar]
- Kop WJ. Chronic and acute psychological risk factors for clinical manifestations of coronary heart disease. Psychosomatic Medicine. 1999;61(4):476–487. doi: 10.1097/00006842-199907000-00012. [DOI] [PubMed] [Google Scholar]
- Kubzansky LD, Kawachi I, Spiro A, Weiss ST, Vokonas PS, Sparrow D. Is worrying bad for your heart? A prospective study of worry and coronary heart disease in the Normative Aging Study. Circulation. 1997;95:818–824. doi: 10.1161/01.cir.95.4.818. [DOI] [PubMed] [Google Scholar]
- Lai JY, Linden W. Gender, anger expression style, and opportunity for anger release determine cardiovascular reaction to and recovery from anger provocation. Psychosomatic Medicine. 1992;54:297–310. doi: 10.1097/00006842-199205000-00006. [DOI] [PubMed] [Google Scholar]
- Larson MR, Langer AW. Defensive hostility and anger expression: Relationship to additional heart rate reactivity during active coping. Psychophysiology. 1997;34:177–184. doi: 10.1111/j.1469-8986.1997.tb02129.x. [DOI] [PubMed] [Google Scholar]
- Lazarus RS, Folkman S. Coping and adaptation. In: Gentry WD, editor. Handbook of behavioral medicine. New York: Guilford Press; 1984. pp. 282–325. [Google Scholar]
- Lewin K. Field theory in social science: Selected theoretical papers. Oxford, UK: Harpers; 1951. [Google Scholar]
- Llabre MM, Spitzer SB, Saab PG, Ironson GG, Schneiderman M. The reliability and specificity of delta versus residualized change as measures of cardiovascular reactivity to behavioral challenges. Psychophysiology. 1991;28(6):701–711. doi: 10.1111/j.1469-8986.1991.tb01017.x. [DOI] [PubMed] [Google Scholar]
- Malliani A. The pattern of sympathovagal balance explored in the frequency domain. News in Physiological Sciences. 1999;14:111–117. doi: 10.1152/physiologyonline.1999.14.3.111. [DOI] [PubMed] [Google Scholar]
- Malliani A, Pagani M, Lombardi F, Cerutti S. Cardiovascular neural regulation explored in the frequency domain. Circulation. 1991;84:482–492. doi: 10.1161/01.cir.84.2.482. [DOI] [PubMed] [Google Scholar]
- Matthews KA, Owens JF, Kuller LH, Sutton-Tyrell K, Jansen-McWilliams L. Are hostility and anxiety associated with carotid atherosclerosis in healthy postmenopausal women? Psychosomatic Medicine. 1998;60:633–638. doi: 10.1097/00006842-199809000-00021. [DOI] [PubMed] [Google Scholar]
- Mente A, Helmers KF. Defensive hostility and cardiovascular responses to stress in young men. Personality and Individual differences. 1999;27:683–694. [Google Scholar]
- Miller TQ, Smith TW, Turner CW, Guijaro ML, Hallet AJ. A meta-analytic review of research on hostility and physical health. Psychological Bulletin. 1996;119:332–48. doi: 10.1037/0033-2909.119.2.322. [DOI] [PubMed] [Google Scholar]
- Neumann SA, Waldstein SR, Sollers JJ, Thayer JF, Sorkin JD. Hostility and distraction have differential influences on cardiovascular recovery from anger recall in women. Health Psychology. 2004;23(6):631–640. doi: 10.1037/0278-6133.23.6.631. [DOI] [PubMed] [Google Scholar]
- Obrist PA. Cardiovascular Psychophysiology. New York, NY: Plenum; 1981. [Google Scholar]
- Pagani M, Lombardi F, Guzzetti S, Rimoldi O, Furlan R, Pizzinelli P, Sandrone G, Malfatto G, Dell’Orto S, Piccaluga E, Turiel M, Baselli G, Cerutti S, Malliani A. Power spectral analysis of heart rate and arterial pressure variabilities as a marker of sympatho-vagal interaction in man and conscious dog. Circulation Research. 1986;59:178–193. doi: 10.1161/01.res.59.2.178. [DOI] [PubMed] [Google Scholar]
- Pieper S, Brosschot JF, van der Leeden R, Thayer JF. Cardiac effects of momentary assessed worry episodes and stressful events. Psychosomatic Medicine. 2007;69:901–909. doi: 10.1097/PSY.0b013e31815a9230. [DOI] [PubMed] [Google Scholar]
- Pomeranz B, MaCaulay RJB, Caudill MA, Kutz I, Adam D, Gordon D, Kilborn KM, Barger AC, Shannon DC, Cohen RJ, Benson H. Assessment of autonomic function in humans by heart rate spectral analysis. American Journal of Physiology. 1985;248:H151–H153. doi: 10.1152/ajpheart.1985.248.1.H151. [DOI] [PubMed] [Google Scholar]
- Porges SW. The polyvagal perspective. Biological Psychology. 2007;74:116–143. doi: 10.1016/j.biopsycho.2006.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powch IG, Houston BK. Hostility, anger-in and cardiovascular reactivity in white women. Health Psychology. 1996;15(3):200–208. doi: 10.1037//0278-6133.15.3.200. [DOI] [PubMed] [Google Scholar]
- Schekelle RB, Gale M, Ostfeld AA, Paul O. Hostility, risk of coronary heart disease, and mortality. Psychosomatic Medicine. 1983;45:109–14. doi: 10.1097/00006842-198305000-00003. [DOI] [PubMed] [Google Scholar]
- Shapiro D, Goldstein IB, Jamner LD. Effects of anger/hostility, defensiveness, gender, and family history of hypertension on cardiovascular reactivity. Psychophysiology. 1995;32:425–435. doi: 10.1111/j.1469-8986.1995.tb02093.x. [DOI] [PubMed] [Google Scholar]
- Shen BJ, Stroud LR, Niaura R. Ethnic differences in cardiovascular responses to laboratory stress: A comparison between Asian and White Americans. International Journal of Behavioral Medicine. 2004;11(3):181–186. doi: 10.1207/s15327558ijbm1103_7. [DOI] [PubMed] [Google Scholar]
- Sherwood A, Royal SA, Hutcheson JS, Turner JR. Comparison of impedence cardiographic measurements using band and spot electrodes. Psychophysiology. 1992;29:734–741. doi: 10.1111/j.1469-8986.1992.tb02051.x. [DOI] [PubMed] [Google Scholar]
- Sirois BC, Burg MM. Negative emotion and coronary heart disease. Behavior Modification. 2003;27(1):83–102. doi: 10.1177/0145445502238695. [DOI] [PubMed] [Google Scholar]
- Smith JL, Ruiz JM. Interpersonal orientation in context: Correlates and effects of interpersonal complementarity on subjective and cardiovascular experiences. Journal of Personality. 2007;75(4):679–708. doi: 10.1111/j.1467-6494.2007.00453.x. [DOI] [PubMed] [Google Scholar]
- Smith TW, Frohm K. What’s so unhealthy about hostility? Construct validity and psychosocial correlates to the Cook & Medley hostility scale. Health Psychology. 1985;4:503–520. doi: 10.1037//0278-6133.4.6.503. [DOI] [PubMed] [Google Scholar]
- Spielberger CD. State-Trait Anger Expression Inventory-2: Professional Manual. Florida: Psychological Assessment Resources, Inc; 1999. [Google Scholar]
- Spielberger CD, Johnson EH, Russell SF, Crane RJ, Jacobs GA, Warden TJ. The experience and expression of anger: Construction and validation of an anger expression scale. In: Chesney MA, Rosenman RH, editors. Anger and hostility in cardiovascular and behavioral disorders. Washington, DC: Hemisphere; 1985. pp. 5–30. [Google Scholar]
- Stoney CM. The role of reproductive hormones in cardiovascular and neuroendocrine function under behavioral stress. In: Turner RJ, editor. Individual differences in cardiovascular responses to stress. New York: Plenum; 1992. pp. 147–163. [Google Scholar]
- Stoney CM, Hughes JW, Kuntz KK, West SG, Thornton LM. Cardiovascular stress responses among Asian Indian and European American women and men. Annals of Behavioral Medicine. 2002;24(2):113–121. doi: 10.1207/S15324796ABM2402_08. [DOI] [PubMed] [Google Scholar]
- Suarez EC, Kuhn CM, Schanberg SM, Williams RB, Zimmerman EA. Neuroendocrine, cardiovascular, and emotional responses of hostile men: The role of interpersonal challenge. Psychosomatic Medicine. 1998;60(1):78–88. doi: 10.1097/00006842-199801000-00017. [DOI] [PubMed] [Google Scholar]
- Suls J, Wan CK. The relationship between trait hostility and cardiovascular reactivity: A quantitative review and analysis. Psychophysiology. 1993;30(6):615–626. doi: 10.1111/j.1469-8986.1993.tb02087.x. [DOI] [PubMed] [Google Scholar]
- Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Heart rate variability: Standards of measurement, physiological interpretation, and clinical use. Circulation. 1996;93:1043–1065. [PubMed] [Google Scholar]
- Thayer JF, Friedman BH, Borkovec TD. Autonomic characteristics of generalized anxiety disorder and worry. Biological Psychiatry. 1996;39:255–266. doi: 10.1016/0006-3223(95)00136-0. [DOI] [PubMed] [Google Scholar]
- Vella EJ, Friedman BH. Autonomic characteristics of defensive hostility: Reactivity and recovery to active and passive stressors. International Journal of Psychophysiology. 2007;66:95–101. doi: 10.1016/j.ijpsycho.2007.03.014. [DOI] [PubMed] [Google Scholar]
- Wilcox S, Bopp M, Wilson DK, Fulk LJ, Hand GA. Race differences in cardiovascular and cortisol responses to an interpersonal challenge in women who are family caregivers. Ethnicity and Disease. 2005;15(1):17–24. [PubMed] [Google Scholar]
- Willemsen GHM, De Geus EJC, Klaver CHAM, Van Doornen LJP, Carroll D. Ambulatory monitoring of the impedance cardiogram. Psychophysiology. 1996;33:184–193. doi: 10.1111/j.1469-8986.1996.tb02122.x. [DOI] [PubMed] [Google Scholar]