Fig. 1. The 120-kd form of FGFR2 is the most abundant tyrosine phosphorylated protein in OLs.
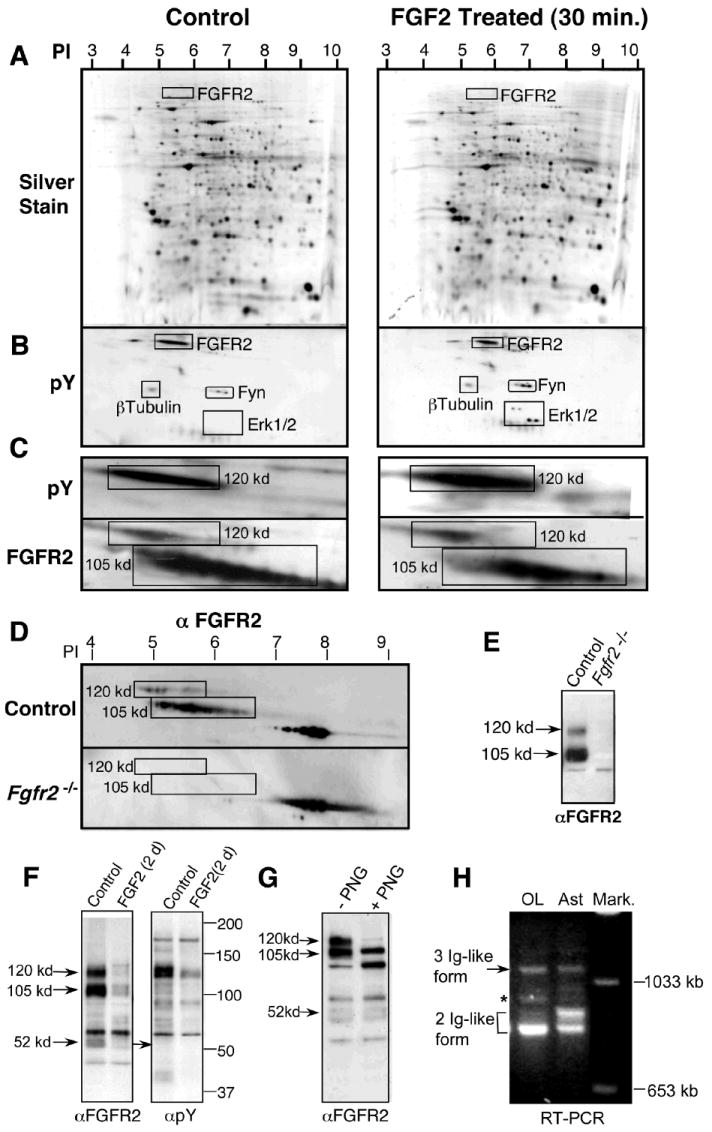
Mature OLs treated with FGF2 (30 min) were analyzed by 2D PAGE (A-C). Equal amounts (350 ug) of total protein separated on each gel were silver stained (A) or immunoblotted with anti-phosphotyrosine (pY) (B, C) and re-probed with anti-FGFR2 (C), showing that the tyrosine-phosphorylated 120-kd band is a form of FGFR2 and that it is phosphorylated even in the absence of FGF2. β-Tubulin, Fyn, and MAPK also are identified as landmarks. Myelin from control and Fgfr2 -/- mice was analyzed by 2D (D) and 1D (E) PAGE and immunolabeled with anti-FGFR2. The 120-kd and105-kd proteins that correspond to the two elongated spots in OLs (C) are present in controls, but not in Fgfr2 -/- myelin, confirming that these are forms of FGFR2. Spot (pI, 7-9) present in both mutant and controls is nonspecific. (F) 1D PAGE of OLs untreated or treated with FGF2 for two days (2d), immunoblotted with anti-FGFR2 and anti-pY, shows that concomitant to the expected downregulation of FGFR2 by 2-d FGF2 treatment, the tyrosine-phosphorylated 120-kd band also is downregulated, further confirming its identity as a form of FGFR2. (G) OL proteins were deglycosylated with PNGase F (PNG) and immunoblotted with anti-FGFR2, showing that both the 105- and 120-kd bands are N-glycosylated equally, evident by similar decreases in their sizes following PNGase treatment compared with untreated. (H) Total RNA samples from OLs or astrocytes (Ast) analyzed by RT-PCR showed amplification of specific DNA fragments indicative of the presence of both the two- and three-Ig-like domain isoforms of FGFR2. Representative experiments of four or more are shown.
